Published online May 21, 2007. doi: 10.3748/wjg.v13.i19.2703
Revised: March 5, 2006
Accepted: March 26, 2007
Published online: May 21, 2007
AIM: To determine the relationship between biliary stricture and pigment gallstone formation, and the prevention of pigment gallstones with medicine.
METHODS: One hundred and eighteen male guinea pigs were randomly divided into four groups: stricture group (S, n = 30) underwent partial ligation of common bile duct, and fed on regular chow; S plus medicine group (S+M, n = 27) underwent the same operation but fed on medicinal chow (0.3 g chenodeoxycholic acid, 0.5 g glucurolactone, and 0.5 g aspirin were mixed up in 1.2 kg regular chow); medicinal control group (C+M, n = 30) was free of operation, and fed on medicinal chow; and control group (C, n = 31) was free of operation and fed on regular chow. One week later, laparotomy was performed, and the bile of gallbladder was collected, measured, and cultured.
RESULTS: Gallstones were identified. Pigment gallstones were induced by biliary stricture in 95% (22/23) of S group. In the S+M group, the incidence of gallstone was reduced to 55% (11/20, vs S group, P < 0.01). The changes of indirect bilirubin and ionized calcium in the bile were consistent with gallstone incidences.
CONCLUSION: Biliary stricture can cause pigment gallstone formation in guinea pigs, and the medicines used can lower the incidence of gallstones. The bilirubin and ionized calcium play important roles in pigment gallstone formation.
- Citation: Xu Z, Ling XF, Zhang WH, Zhou XS. Can pigment gallstones be induced by biliary stricture and prevented by medicine in Guinea pigs? World J Gastroenterol 2007; 13(19): 2703-2706
- URL: https://www.wjgnet.com/1007-9327/full/v13/i19/2703.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i19.2703
Pigment gallstone is very common in Asia, and its treatment, especially for intrahepatic ones, is a very troublesome problem in biliary surgery[1,2]. The therapeutic challenges include difficulty in clearing stones and a high rate of stone recurrence. Hepatolithiasis coexisting with intrahepatic biliary stricture is particularly difficult to manage[3]. How to prevent pigment gallstone formation with medicine is significant to treat and prevent this disease.
Pigment gallstone formation is a very complex mechanism, and many aspects have not been as fully studied as those of cholesterol. According to the pathogenesis of pigment gallstone formation, Maki emphasized the enhancement of bacterial beta-glucuronidase (β-G) activity and resultant increase of unconjugated bilirubin (UCB) concentration in bile, which combined with Ca++ to form calcium bilirubinate[4]. This opinion was commonly accepted in the 1970s and still studied in the 2000s[5]. But clinically, it is known that biliary stricture itself can cause pigment gallstone formation without biliary infection. This fact can not be fully explained by Maki’ s theory.
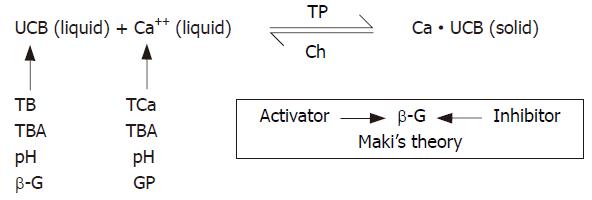
In the previous experiments, the conditional constant of the solubility product (K’sp) of calcium bilirubinate in water solution was measured and confirmed[6,7]. This suggested that there is an equilibrium between the precipitation and dissolution of calcium bilirubinate. In this equilibrium, UCB and Ca++ are key links in the precipitation of “bilirubin-calcium compound” (Figure 1). Other factors,such as total calcium, total bilirubin, β-G, total bile acid, cholesterol, glycoprotein, play their roles by acting on the key links in this process. This theory could explain the clinical phenomena more reasonably. However, this theory was established according to the results in vitro, and could not be validated in vivo.
Medicines were used to prevent pigment gallstone formation. They contained chenodeoxycholic acid, glucurolactone, and aspirin. Chenodeoxycholic acid was reported to reduce pigment gallstone formation in hamster by preventing precipitation of calcium bilirubinate[8-10]. Glucurolactone could inhibit β-G activity, and decrease the precipitation of Ca++ and bilirubin[6,7]. Aspirin could prevent cholesterol gallstone formation by inhibiting the synthesis of glucoprotein in prairie dogs[11].
The aim of this study was to determine the role of biliary stricture in pigment gallstone formation and the possibility of prevention with medicines, and at the same time, to validate the equilibrium theory of pigment gallstone formation in vivo.
One hundred and eighteen male guinea pigs (weighing 300-600 g, purchased from Medical Experimental Animal Center of Peking University, Beijing, China) were randomly divided into four groups.
Stricture (S) group: Guinea pigs (n = 30) were fasted for 16 hours, and anaesthetized with intraperitoneal sodium pentobarbital (30 mg/kg). After laparotomy, common bile duct (CBD) was exposed. A 5-gauge dull needle (0.5 mm in diameter) was placed beside CBD and tied together with 1-0 suture. After withdrawing the needle, incomplete stricture of CBD was achieved[12]. Abdominal wall was closed. The animals were fed with regular chow (made by Animal Chow Factory, Chaoyang District, Beijing) postoperatively.
Stricture plus medicine (S+M) group: Guinea pigs (n = 27) underwent the same operation as S group but fed with medicinal chow (0.3 g chenodeoxycholic acid, 0.5 g glucurolactone, and 0.5 g aspirin were mixed up in 1.2 kg regular chow) after operation.
Medicinal control (C+M) group: Animals (n = 30) were free of operation, and fed with medicinal chow.
Control (C) group: Animals (n = 31) were free of operation and fed with regular chow.
One week later, the animals were fasted and anaesthetized as mentioned above, and laparotomy was performed. Gallbladder bile was aspirated with a 20-gauge syringe, measured as the volume of gallbladder and performed in dimmed light as much as possible so as to avoid photoisomerization of bile pigments. Culture for standard bacteria was performed. Bile samples were immediately analyzed for Ionized calcium (Ca++) with a selective iron analyzer Model PXS-201 (Shanghai 2nd Analytical Instrument Works, Shanghai, China), equipped with saturated calomel electrode (Shanghai Tienkuang Device Works, Shanghai, China) and calcium ion selective polyvinyl chloride (PVC) membrane electrode (Suzhou Standard Metrological Experimental Factory, Suzhou, China)[6], pH value with a Model PHS-3 digital standard pH meter (Shanghai 2nd Analytical Instrument Works, Shanghai, China)[6], total bilirubin (TB) and indirect bilirubin (IB) which were used to represent UCB[13] and β-G[14]. Bile samples were then centrifuged at 3500 r/min for 10 min, and the supernatant was aspirated and stored at -20°C for measurement of total calcium (TCa)[15], glycoprotein (GP)[16], total bile acid (TBA)[17], total phosphorus (TP)[18] and cholesterol (Ch)[19].
Examination of biliary tract and identification of gallstones. Animals were sacrificed. Gallbladder and bile duct were resected and examined. The perimeter of CBD at the upper side of ligation was measured. Gallstones were collected, recorded and identified by qualitative analysis[20].
Quantitative data were expressed as mean ± SD and performed with t (t’) test. Data were analyzed qualitatively with χ2 test. SPSS 11 was used as computer statistical program. A P value of less than 0.05 was considered statistically significant.
Survival rates of guinea pigs are listed in Table 1. At first, CBD was too tightly tied, 11 of 57 animals died of acute hepatic failure because of acute complete stricture of CBD. Two of them died of acute pneumothorax, and one died of abdominal bleeding.
Gallstone incidences are listed in Table 2. Pigment gallstones were induced in 95.7% (22/23) of S group (P < 0.01 vs C group, 0/31), and in S+M group, the incidence of gallstone was reduced to 55.0% (P < 0.01, 11/20 vs S group) in one week, respectively.
Gallbladder volume and perimeter of common bile duct were measured and are listed in Table 3. Partial ligation of CBD evidently increased the gallbladder volume, and dilated the CBD.
Components of gallbladder bile, collected at the end of the first week of the experiment, were analyzed and the results are shown in Table 4. The concentrations of IB and Ca++ in bile were consistent with gallstone incidences. Ten bile samples from S group, nine from S+M group, six from C group, and six from C+M group were cultured for standard bacteria and no bacterium grew.
| Components | Groups | |||
| S | S+M | C+M | C | |
| Ca++ (μmol/L) | 111.7 ± 19.3 (23)b | 93.5 ± 22.8 (20) | 31.1 ± 4.8 (15) | 29.7 ± 7.9 (16)b |
| TCa (μmol/L) | 174.8 ± 81.7 (22) | 176.8 ± 113.3 (16) | 111.9 ± 46.3 (7) | 80.6 ± 29.7 (5)a |
| TB (mg/dL) | 2.73 ± 1.30 (22)a | 1.82 ± 1.03 (17) | 1.09 ± 0.55 (6) | 1.09 ± 0.30 (11)b |
| IB (mg/dL) | 1.30 ± 0.70 (22)a | 0.79 ± 0.63 (17) | 0.84 ± 0.32 (6) | 0.55 ± 0.29 (11)b |
| β-G (μmol/dL) | 938 ± 627 (17) | 809 ± 569 (15) | 1763 ± 933 (12)a | 1178 ± 548 (9) |
| TP (mg/dL) | 6.54 ± 6.53 (22) | 5.99 ± 6.89 (15) | 3.10 ± 2.17 (7) | 3.26 ± 0.60 (4)a |
| TBA (mmol/L) | 14.8 ± 9.2 (22) | 14.4 ± 7.6 (16) | 13.2 ± 4.9 (7) | 9.9 ± 2.4 (5) |
| GP (mg/dL) | 3.05 ± 1.63 (12) | 2.89 ± 1.40 (9) | - | 0.46 ± 0.28 (9)b |
| Ch (mg/dL) | 4.75 ± 2.04 (17) | 3.79 ± 1.66 (13) | 3.68 ± 2.05 (4) | 3.42 ± 0.86 (8)a |
| pH | 8.46 ± 0.14 (9)a | 8.27 ± 0.15 (9) | 8.35 ± 0.12 (10)a | 8.14 ± 0.20 (4)b |
According to the pathogenesis of pigment gallstone formation, Maki emphasized the enhancement of bacterial β-G activity and resultant increase of UCB concentration in bile, which combined with Ca++ to form calcium bilirubinate[4]. This point of view was commonly accepted in the 1970s and still studied in the 2000s[5]. But in this study, pigment gallstone was induced by partial ligation of CBD, gallbladder bile was sterile by standard bacteria culture, and β-G activity in S group was not higher than in C group (P > 0.05, Table 4), whereas the IB (similar to UCB) content in the bile of S group was significantly higher than in C group (P < 0.01, Table 4). Gallstones, containing calcium bilirubinate, were formed in S group (P < 0.01, S vs C groups, Table 2). These facts could not be fully explained by Maki’ s theory.
But if we use the equilibrium theory to explain these facts, it seems reasonable (Figure 1). Incomplete common bile duct stricture did cause increased UCB and Ca++ in S group (P < 0.01, S vs C groups, Table 4). And pigment gallstone was indeed formed in S group (P < 0.01, S vs C groups, Table 2). These facts were firstly validated that UCB and Ca++ were key links and played very important roles in the process of pigment gallstone formation in vivo. Other factors played their roles by acting on UCB and Ca++. β-G and its inhibitors or activators, as Maki had indicated, were certainly responsible for UCB concentration[4]. Besides, TB (similar to conjugated bilirubin, CB) was increased in S group (P < 0.01, S vs C groups, Table 4), and could supply UCB material, and hydrolyze into UCB even in the absence of β-G[14]. pH was alkalized in S group (P < 0.01, S vs C groups, Table 4), and alkalization of the bile would catalyze the hydrolysis from CB into UCB[21]. TCa was also increased in S group (P < 0.01, S vs C groups, Table 4), and could supply Ca++ material and enhance the concentration of Ca++. GP was increased in S group (P < 0.01, S vs C groups, Table 4), and was capable of promoting calcium bilirubinate precipitation, especially in the presence of high Ca++ content[13,14]. It was suggested that GP enhanced the equilibrium of calcium bilirubinate to the side of precipitation by means of trapping which already formed precipitate and prevented from redissolution (Figure 1). pH and bile salts would determine the form of UCB in bile, thus determining the activity of UCB[22]. TP was increased in S group (P < 0.05, S vs C groups, Table 4), facilitated UCB and Ca++ to form calcium bilirubinate, and promoted pigment gallstone formation. Ch was increased in S group (P < 0.05, S vs C groups, Table 4), and could inhibit the precipitation of calcium bilirubinate, but its effect was failed because its function was too week as compared with other elements such as UCB, Ca++, GP, and so on. In this equilibrium (Figure 1), bile salts worked as a buffer of Ca++. They were able to form soluble compound by combining with Ca++, and resulted in reduction of Ca++ activity. TBA was not increased in S group (P > 0.05, S vs C groups, Table 4), and its effect was too weak to inhibit the precipitation of calcium bilirubinate in this study. As a result of UCB or Ca++ activity and concentration alteration, the equilibrium of calcium bilirubinate precipitation and dissolution would be changed by these factors (Figure 1).
Partial ligation of CBD evidently increased the gallbladder volume, and dilated the CBD in S group (P < 0.01, S vs C group, Table 3). It was indicated that the animal model of incomplete stricture of CBD was successful[12]. It is verified that incomplete stricture of CBD could induce pigment gallstone formation[23].
At the beginning of partial ligation of CBD, medicines were used to prevent pigment gallstone formation. They contained chenodeoxycholic acid, glucurolactone, and aspirin. These medicines decreased the ratio of pigment gallstone formation in strictured animals from 95.7% (22/23) in S group to 55% (11/20) in S+M group (P < 0.01, Table 2). At the same time, the concentration of Ca++ was significantly decreased in S+M group (P < 0.01, S vs S+M group, Table 4), and the content of TB, IB, and pH was decreased in S+M group (P < 0.05, S vs S+M group, Table 4). These results also indicated that Ca++ and UCB were the key links in the process of pigment gallstone formation (Figure 1) verified in animal models[6]. β-G activity was slightly decreased in S+M group compared with S group, but it was not statistically significant (P > 0.05, S vs S+M group, Table 4)[24], whereas the content of IB was reduced significantly (P < 0.01, S vs S+M group, Table 4). It could not be well explained that the decrease of concentration of IB was caused by the reduction of β-G activity[4]. The significant decrease of pH (S vs S+M group, P < 0.05, Table 4) might reduce the hydrolysis from CB into UCB[7,21]. The significant decrease of TB might reduce the source of IB. Although content of TBA in S+M group was not higher than that in S group (P > 0.05, Table 4), the content of Ca++ in S+M group was significantly less than in S group (P < 0.01, Table 4), and concentrations of TCa in both S+M and S groups were almost the same (P > 0.05, Table 4). It was indicated that Ca++ buffer capacity of bile in S+M group was enhanced by chenodeoxycholic acid administration[8-10]. GP content in the bile of S+M group was slightly less than that of S group, but had no significance (P > 0.05, Table 4). It seemed that the effectiveness of aspirin in decreasing the GP concentration was uncertain in this study. The reason might be that the dosage administered was not enough compared with Lee’s report (25 mg/kg vs 100 mg/kg)[11]. But the important role of GP in promoting pigment gallstone formatio [25] was confirmed by the fact that the significant elevation of GP was consistent with the high pigment gallstone incidence in S group (Tables 2 and 4), and that within S+M group, GP in the bile of animals with stone (3.84 ± 0.40 mg/dL, n = 5) was higher than in those without stone (P < 0.01, 1.69 ± 1.26 mg/dL, n = 4, t = 3.648). Medicines used in this study had compound effects to reduce pigment gallstone formation by decreasing the concentrations of Ca++ and UCB, and could be used as preventive medicines against pigment gallstone formation in the future.
There were no differences in IB, Ca++ and gallstone incidences between C+M and C groups (P > 0.05, Tables 2 and 4). It seemed that the medicines used mainly resisted the pathophysiological changes caused by partial ligation of CBD, but did not act on normal animals.
S- Editor Liu Y L- Editor Ma JY E- Editor Liu Y
| 1. | Chen DW, Tung-Ping Poon R, Liu CL, Fan ST, Wong J. Immediate and long-term outcomes of hepatectomy for hepatolithiasis. Surgery. 2004;135:386-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 95] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 2. | Paulo H, Marcel MCC. Primary intrahepatic lithiasis. Gen Surg. 2001;18:51-55. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 3. | Jeng KS, Sheen IS, Yang FS. The benefits of a second transhepatic route in failed percutaneous management of difficult intrahepatic biliary strictures with recurrent hepatolithiasis. Surg Laparosc Endosc Percutan Tech. 2001;11:170-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 4. | Maki T. Pathogenesis of calcium bilirubinate gallstone: role of E. coli, beta-glucuronidase and coagulation by inorganic ions, polyelectrolytes and agitation. Ann Surg. 1966;164:90-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 325] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 5. | Leung JW, Liu YL, Leung PS, Chan RC, Inciardi JF, Cheng AF. Expression of bacterial beta-glucuronidase in human bile: an in vitro study. Gastrointest Endosc. 2001;54:346-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Fu XP, Zhou XS, Zhang WH, Deng SQ, Shao XM. "Bilirubin-calcium compound" precipitation and the effect of bile salts on it. The pathogenesis of pigment gallstone. Chin Med J (Engl). 1985;98:728-738. [PubMed] |
| 7. | Moore EW, Vérine HJ. Pathogenesis of pancreatic and biliary CaCO3 lithiasis: the solubility product (K'sp) of calcite determined with the Ca++ electrode. J Lab Clin Med. 1985;106:611-618. [PubMed] |
| 8. | Setoguchi I, Cohen I, Mosbach EH, Soloway RD, Rizigalinski B. A new hamster model of pigment cholelithiasis (abstr). Gastroenterology. 1985;88:1693. |
| 9. | Ostrow JD, Celic L. Bilirubin chemistry, ionization and solubilization by bile salts. Hepatology. 1984;4:38S-45S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Kaplan SA, Lakshminarayanaiah N, Chun P, Trotman BW. Bile salt prevent precipitation of calcium bilirubinate in vitro (abstr). Gastroenterology. 1984;86:1352. |
| 11. | Lee SP, Carey MC, LaMont JT. Aspirin prevention of cholesterol gallstone formation in prairie dogs. Science. 1981;211:1429-1431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 167] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | Shiesh SC, Chen CY, Lin XZ, Liu ZA, Tsao HC. Melatonin prevents pigment gallstone formation induced by bile duct ligation in guinea pigs. Hepatology. 2000;32:455-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Hogg CK, Meites S. A modification of the Malloy and Evelyn procedure for the micro-determination of total serum bilirubin. Am J Med Technol. 1959;25:281-286. |
| 14. | Fishman WH, Kato K, Anstiss CL, Green S. Human serum beta-glucuronidase; its measurement and some of its properties. Clin Chim Acta. 1967;15:435-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 154] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Dawes LG, Rege RV. Calcium and calcium binding in human gallstone disease. Arch Surg. 1990;125:1606-1609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 16. | Brown RE, Jarvis KL, Hyland KJ. Protein measurement using bicinchoninic acid: elimination of interfering substances. Anal Biochem. 1989;180:136-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 498] [Cited by in RCA: 480] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 17. | Turley SD, Dietschy JM. Re-evaluation of the 3 alpha-hydroxysteroid dehydrogenase assay for total bile acids in bile. J Lipid Res. 1978;19:924-928. [PubMed] |
| 18. | Bartlett GR. Phosphorus assay in column chromatography. J Biol Chem. 1959;234:466-468. [PubMed] |
| 19. | Rudel LL, Morris MD. Determination of cholesterol using o-phthalaldehyde. J Lipid Res. 1973;14:364-366. [PubMed] |
| 20. | Zhou XS, Wang SS, Zhang KL, Zhang WH, Chen HH, Wu JG, Shen GR, Gao YY, Lin JH, Zhu SG. Pigment gallstones study. Chin Med J (Engl). 1982;95:905-911. [PubMed] |
| 21. | Ostrow JD, Murphy NH. Isolation and properties of conjugated bilirubin from bile. Biochem J. 1970;120:311-327. [PubMed] |
| 22. | Berman MD, Koretsky AP, Carey MC. Influence of pH on the solubility of unconjugated bilirubin (UCB) in artificial bile solution (abstr). Gastroenterology. 1980;78:1141. |
| 23. | Terada T, Terai T, Yamawaki T. Marked diffuse dilations of the biliary tree associated with intrahepatic calculi, biliary sludges and a mucinous cyst of the pancreatic head in a 99-year-old woman. Pathol Int. 2003;53:563-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 24. | Bengochea L, Ghanem C, Perazzo JC, Ghisolfi C, Marabotto L, Acevedo C, Mino J, Lemberg A, Rubio M. Drug glucuronidation and hepatic lipid microsomal membrane profile in cholestatic rats followed paracetamol intoxication. Pharmacol Res. 1999;40:369-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 25. | Zhang H, Tsang TK, Jack CA. Bile glycoprotein mucin in sludge occluding biliary stent. J Lab Clin Med. 2003;142:58-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |