Published online May 14, 2007. doi: 10.3748/wjg.v13.i18.2529
Revised: February 18, 2007
Accepted: March 21, 2007
Published online: May 14, 2007
Magnetic resonance cholangiopancreatography (MRCP) is being used with increasing frequency as a noninvasive alternative to diagnostic retrograde cholangiopancreatography (ERCP). The aim of this pictorial review is to demonstrate the usefulness of MRCP in the evaluation of pancreatic and biliary system disorders. Because the recently developed techniques allows improved spatial resolution and permits imaging of the entire pancreaticobiliary tract during a single breath hold, MRCP is of proven utility in a variety of pancreatic and biliary disorders. It uses MR imaging to visualize fluid in the biliary and pancreatic ducts as high signal intensity on T2 weighted sequences and is the newest modality for pancreatic and biliary duct imaging. Herein, we present the clinical applications of MRCP in a variety of pancreaticobiliary system disorders and conclude that it is an important diagnostic tool in terms of imaging of the pancreaticobiliary ductal system.
- Citation: Halefoglu AM. Magnetic resonance cholangiopancreatography: A useful tool in the evaluation of pancreatic and biliary disorders. World J Gastroenterol 2007; 13(18): 2529-2534
- URL: https://www.wjgnet.com/1007-9327/full/v13/i18/2529.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i18.2529
Magnetic resonance cholangiopancreatography (MRCP) is a noninvasive imaging technique that accurately depicts the morphological features of the biliary and pancreatic ducts. By using heavily T2 weighted sequences, the signal of static or slow-moving fluid-filled structures such as the bile and pancreatic ducts is greatly increased, resulting in increased duct-to-background contrast. Recent studies have shown that MRCP is comparable with invasive retrograde cholangiopancreatography (ERCP) for diagnosis of extrahepatic bile duct and pancreatic duct abnormalities such as choledocholithiasis[1-3], malignant obstruction of the bile and pancreatic ducts[1,2], congenital anomalies[1,4], and chronic pancreatitis[5,6]. Common indications for MRCP usually include unsuccessful ERCP or a contraindication to ERCP and the presence of biliary-enteric anastomoses. (e.g., choledochojejunostomy, Billroth 2 anastomosis). In some institutions, MRCP is becoming the initial imaging tool for the biliary system, with ERCP reserved for only therapeutic indications. In this article we present clinical applications of MRCP in the pancreaticobiliary system pathologies including choledocholithiasis, biliary strictures, chronic pancreatitis, benign and malignant pancreatic neoplasms, pancreatic pseudocysts, congenital abnormalities and postsurgical biliary tract alterations.
During the MRCP examinations, respiratory motion induced blurring has limited demonstration of the biliary and pancreatic ductal system and different approaches have been considered to overcome this problem. As a result, the technical history of MRCP parallels the evolution of progressively faster T2 weighted imaging sequences, i.e., from gradient-echo, to fast spin echo (FSE), to single-shot fast spin-echo (SSFSE)[7]. SSFSE is a recently developed ultrafast T2 weighted sequence, which allows subsecond slice acquisition. This largely overcomes the problem of motion artifact in MRCP, because physiologic motion is "frozen", and imaging of the biliary and pancreatic ducts can be performed in a single breath-hold[8].
SSFSE is the current sequence of choice for MRCP, because it essentialy eliminates the problem of motion artifact, and because of greater contrast-to-noise ratio and increased spatial resolution when compared with FSE or gradient-echo-based T2 weighted sequences[9]. MRCP is usually performed by using SSFSE software and both a thick-collimation (single-section) and thin-collimation (multisection) technique with a torso phased-array coil. The coronal plane is used to provide a cholangiographic display, and the axial plane is used to evaluate the pancreatic duct and the distal common bile duct. In addition we perform three-dimensional reconstruction by using a maximum intensity projection (MIP) algorithm on the thin-collimation source images. Although the thick-collimation and MIP images more closely resemble conventional cholangiograms and are familiar to many clinicians, spatial resolution is degraded because of volume-averaging effects. Diagnostic decisions are usually made on the basis of the thin-collimation source images, however, MIP images often allow depiction of a greater length of duct on a single image than on any one thin-collimation source image. In addition, MIP images are useful in the three-dimensional depiction of ductal anatomy and in planning surgical procedures and radiation therapy. On the other hand, the source images, which provide greater spatial resolution, must be carefully scrutinized so as not to overlook small luminal filling defects and strictures, which may be obscured on the thicker-collimation images.
In a study of 108 patients with a variety of biliary and pancreatic diseases, bile duct stenoses, dilatation, and stones were all better seen on source thin slices than on either MIP reconstruction or single thick slice MRCP[10].
Another disadvantage of such techniques is that periductal structures are deliberately excluded from the final images, even though extraluminal detail may be of critical importance, as in the assessment of neoplastic duct obstruction.
Patients should be fasting for approximately 4-6 h prior to the exam to promote gallbladder filling and gastric emptying. Some authors have advocated the use of glugacon to suspend peristalsis, but use of rapid pulse sequences obviated this requirement. We do not need an exogenous contrast to demonstrate the pancreatic and biliary ducts. The long T2 of fluid compared with that of surrounding soft tissues and of calculi provides sufficient intrinsic contrast. Administration of a negative oral contrast helps reduce the signal intensity from overlapping fluid in the stomach and duedonum.
MRCP is comparable with ERCP in detection of choledo-cholithiasis and superior to CT and US[2,3]. Numerous studies have shown sensitivities of 81%-100% and specificities of 85%-100%[11].
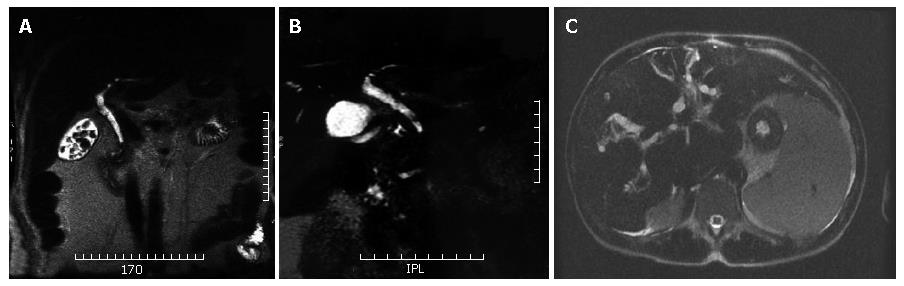
Biliary stones, independently of calcium content, present almost always a low signal intensity on MR images. Therefore, the stone is identified as a round or oval- shaped "filling defect" within the common bile duct (CBD), surrounded by the high signal intensity bile (Figure 1A). Although spatial resolution of MRCP is lower than ERCP, the higher contrast resolution allows 2 to 3 mm stones to be easily detected[12].
It is crucial to scrutinize the thin, source images because the sensitivity for detection of small stones decreases with an increase in section thickness owing to volume averaging of high signal intensity bile surrounding the stone.
Nevertheless, different pitfalls can be observed which requires correct identification in order to avoid wrong diagnoses. They are represented by: a-artefacts on MIP reconstructed images, b-CBD completely filled with stones, c-pneumobilia, and, d-differential diagnoses between air bubles and small stones.
Benign biliary strictures are the result of surgical injury in 90%-95% of cases (laparoscopic cholecystectomy, gastric and hepatic resection, biliary-enteric anastomoses, post liver transplantation), external penetrating or blunt trauma, inflammation associated with lithiasis, chronic pancreatitis, stricture of the papillary region, toxic or ischaemic lesion of the hepatic artery or primary infection such as in primary sclerosing cholangitis[13].
MRCP has been shown to be comparable with ERCP in demonstrating the location and extent of strictures of the extrahepatic bile duct (Figure 1B), with sensitivities of 91%-100%[14]. However, the accuracy in detections of strictures of the intrahepatic bile ducts is under investigation.
Sclerosing cholangitis is a fibrosing, inflammatory process of the bile ducts that leads to sclerosis and stenosis of both intrahepatic and extrahepatic bile ducts.
Strictures are multifocal and alternate with slight dilatation or normal-caliber bile ducts, producing a beaded or "pruned tree" appearence (Figure 1C).
Because MRCP is not as sensitive as ERCP to the early peripheral ductal changes of sclerosing cholangitis, it should be reserved for diagnosis of complications or follow up of more advanced cases.
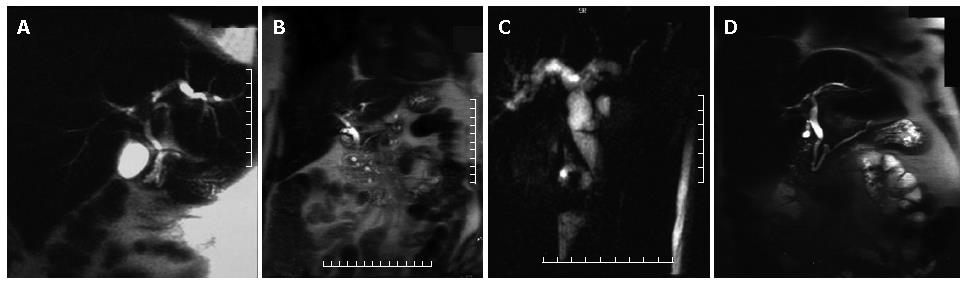
Cholangiocarcinoma may present as a stricture, involving the CBD (30%-36%), the common hepatic duct (15%-30%), the biliary bifurcation, with the typical aspect of Klatskin tumour (10%-26%), and the intrahepatic ducts (8%-13%) with no evidence of mass lesion or as a nodular process with intrahepatic solid mass. The MRCP features are a sudden biliary obstruction with dilatation of bile ducts above. In the case of Klatskin tumours, information regarding the involvement only of the right or left biliary system or both can be easily obtained, with important consequences on therapeutic approach (Figure 2A). Similiar to other neoplastic lesions, conventional MR images are needed for correct lesion identification and staging. In particular, for cholangiocarcinoma, T1 weighted images after contrast medium injection can be very helpful in correct identification of the lesion and of its relationship with surrounding organs, although in the case of stenosing lesion, no expansile process is usually identified.
Initial studies suggest that iatrogenic biliary injuries are well demonstrated at MRCP[15]. Bile leaks result in accumulation of fluid, usually in the subhepatic space, which is readily detected at MRCP. But MRCP can not determine if a leak is active. Recently, there have been reports of use of selective hepato-biliary contrast agent, mangafodipir. This is metabolized by hepatocytes and excreted in bile. This agent may prove useful in noninvasive detection of active bile leaks[16], but prospective trials have not yet been published.
A number of congenital variants in biliary duct anatomy are of surgical significance, because they have been shown to increase the risk of bile duct injury during cholecystectomy. Such variants include a low cystic duct insertion, a medial cystic duct insertion, a long parallel course of the cystic and common hepatic ducts, a short cystic duct, and an abberant right posterior sectorial duct draining to the cystic duct or to the common hepatic duct[4].
Using conventional cholangiography as the standart of reference, MRCP had a sensitivity and specificity of 86% and 100% in the diagnosis of cystic duct variants, and 71% and 100% in the diagnosis of aberrant right hepatic duct, respectively[4].
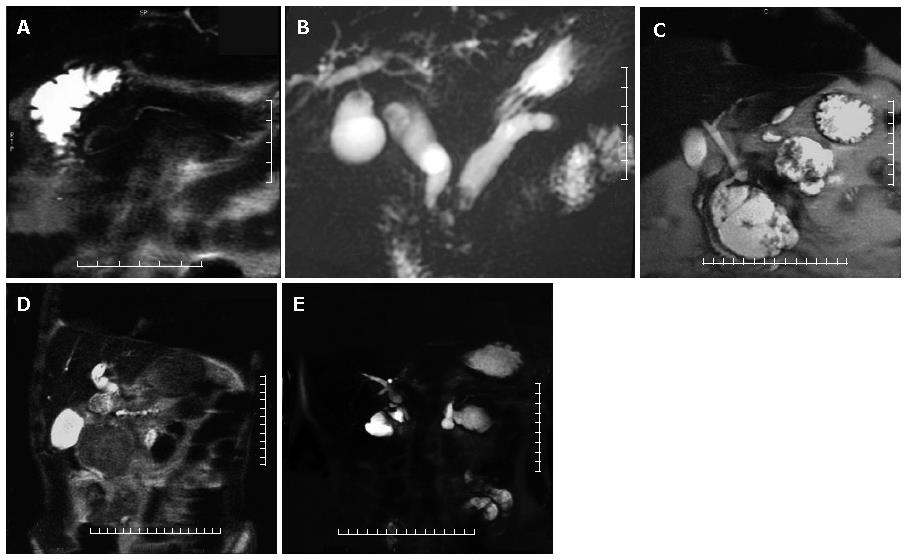
In normal individuals, the main pancreatic duct (duct of Wirsung) drains through the major papilla; this duct is the major drainage route of the pancreas in 91% of individuals. The accessory pancreatic duct (duct of Santorini) drains through the minor papilla and is present in 44% of individuals. Pancreas divisum, the most common anatomic variant of the pancreas, results from failure of fusion of the dorsal and ventral pancreatic ducts and may be associated with an increase prevalance of acute pancreatitis[5]. The larger, dominant dorsal pancreatic duct, which drains the pancreatic tail, body, and superior head, courses anterior to the CBD and drains into the minor papilla separately from the CBD, superior to the major papilla. The smaller ventral duct, which drains the inferior pancreatic head and uncinate process, accompanies the CBD into the major papilla (Figures 2B and 3A). Bret et al[17] reported an accuracy of 100% for MRCP in the diagnosis of pancreas divisum.
The MRCP diagnostic criteria for chronic pancreatitis include duct dilatation, narrowing, stricture, or irregula-rity[7]. Other possible imaging findings are irregularity of pancreatic contour, pseudocysts, and ductal filling defects due to stones, debris or mucinous plugs. In advanced chronic pancreatitis, the duct dilatation is more marked and can be accompanied by CBD dilatation producing "double duct sign" as in the case of pancreatic head carcinoma (Figure 3B).
In chronic pancreatitis intraductal calculi may be seen. These calculi are seen as low signal filling defects surrounded by high signal intensity pancreatic fluid (meniscus sign). In severe pancreatitis, side branches have a "chain of lake" appearance.
Soto et al[18] found the sensitivity of MRCP for dilatation as 87%-100%, for narrowing 75%, and for ductal calculi 100%. The authors conclude that MRCP can accurately demonstrate pancreatic duct abnormalities in chronic pancreatitis.
However, because MRCP is probably not sensitive to the early side-branch changes of chronic pancreatitis, MRCP should be reserved for diagnosis of complications or follow-up of more advanced cases. ERCP is more sensitive to early side-branch changes because of its increased spatial resolution.
Pancreatic pseudocysts are encapsulated fluid collections that may occur in association with acute or chronic pancreatitis. (Figure 3C) MRCP is more sensitive than ERCP in detection of pseudocysts because less than 50% of pseudocysts fill with contrast material at ERCP[19]. However MRCP is less sensitive in demonstrating the site of communication with the pancreatic duct.
Although as many as 60% of pseudocysts may resolve spontaneously, others will become complicated by infection or hemorrhage. MRI and MRCP are useful in demonstrating pseudocysts and possibly their ductal communications as well as in establishing the presence of associated hemorrhage without the risk of infecting the pseudocyst as may occur at ERCP.
Approximately 90% of malignant pancreatic neoplasms are ductal in origin with most being adenocarcinomas. Pancreatic carcinoma is seen as a focal mass in 95% of cases, whereas diffuse involvement of the gland occurs in the remaining 5%[20]. Of these focal carcinomas, 62% are located in the pancreatic head, with the remainder located in the body (26%) and tail (12%) of the pancreas[20].
The MRCP findings of pancreatic carcinoma include encasement and obstruction of the pancreatic duct or bile duct. Dilatation of both ducts constitutes the "double duct sign", which is highly suggestive of but no diagnostic for malignancy[14]. (Figure 2C and Figure 3D) In pancreatic head carcinoma, biliary and pancreatic duct dilatation occurs in 77% of cases, biliary duct dilatation in 9%, and pancreatic duct dilatation in 12%[20].
However, a normal-sized pancreatic duct should not cause this diagnosis to be excluded because the caliber will be normal in up to 20% of patients with pancreatic malignancy causing bile duct obstruction.
In a study of breath-hold SSFSE MRCP in 32 patients with pathologically confirmed neoplastic duct obstruction, the level of obstruction was correctly identified in 27 (84%) and 28 (88%) of the 32 cases by two independent observers, respectively, and the site of underlying tumor was correctly identified in 27 (84%) and 29 (91%) cases[21].
In cases of periampullary carcinoma, apart from the CBD obstruction, high grade obstruction with abrupt termination and mild dilatation of the pancreatic duct is usually present[22] (Figure 2D).
MRCP is also useful in the evaluation of intraductal papillary mucinous tumors. These tumors arise from the epithelium of the main pancreatic duct or side branches. They are slow-growing tumors characterized by pro-duction of large amounts of mucin. Side-branch ductal involvement is typically associated with benign adenomas and a localized cystic parenchymal lesion. Main pancreatic duct involvement alone presents as diffuse duct dilatation, gross mucin production, and micropapillary studding, and is typically associated with malignancy[23,24]. The diagnosis was traditionally made at ERCP. MRCP is now considered superior to ERCP because of its ability to demonstrate the full extent of ductal involvement, particularly when obstructing mucin prevents complete ductal opacification by ERCP[25]. In addition, MRCP can demonstrate main ductal and side branch stenosis and dilatation, associated cystic lesions, and communication between these lesions and the ductal system[26]. Filling defects caused by papillary projections may also be demonstrated.
Pancreatic injuries occur in 2%-12% of patients with blunt abdominal trauma[27]. Disruption of the pancreatic duct is a key prognostic indicator, and early diagnosis is important. Although CT is a sensitive method for detecting parenchymal injury, demonstration of duct injury often requires ERCP[28]. MRCP has recently been advocated as a noninvasive method for diagnosing pancreatic ductal injury[29]. In a series of seven trauma patients reported by Soto et al[30], MRCP accurately demonstrated the status of the duct and the site of duct transection in all patients. Though CT will likely remain the mainstay for diagnosis of pancreatic injury, MRCP shows promise in the planning of therapeutic surgical or endoscopic interventions in this setting[30].
Biliary-enteric anostomoses such as choledocho-jejunostomy, hepaticojejunostomy, and Billroth 2 anostomosis make it difficult or impossible to access the major papilla at endoscopy. In patients with such anastomoses, MRCP is the imaging modality of choice for the work-up of suspected pancreaticobiliary disease.
It has been reported that MRCP is 100% sensitive in detection of anastomotic strictures and 90% sensitive in detection biliary tract stones proximal to the anastomosis[31]. MRCP is also 100% sensitive in demonstrating the choledochojejunal anastomosis after a whipple procedure[1] (Figure 3E).
Close scrutiny of the source images is mandatory because the biliary-enteric anastomosis and stones can be obscured on the thick-section and MIP images by the high signal intensity of the surrounding bile and bowel fluid.
Strictures may be overestimated on the MIP images[31].
A clear advantage of this technique is the lack of invasi-veness. In addition, MRCP is not limited in patients with altered anatomy (choledocho or pancreaticojejunostomy, Billroth 2 etc.) and it is not operator dependent.
The current shortcoming of MRCP is its relatively low spatial resolution which limits the visualization of non-dilated pancreatic duct side branches and characterization of strictures. This makes MRCP inable to assess small duct disease. Examples of small duct disease include subtle intrahepatic duct changes of sclerosing cholangitis, and side branch changes of mild chronic pancreatitis. This limitation is also partially related to the lack of duct distension at MRCP.
In summary, MRCP is a non-invasive important tool in the diagnosis of bilio-pancreatic diseases and has a comparable accuracy to ERCP. Despite relatively low spatial resolution when compared with ERCP the early assessments of diagnostic performance suggest that MRCP can; (1) reliably demonstrate normal and abnormal pancreatic and biliary ducts, (2) accurately diagnose the cause and site of obstruction, (3) be of diagnostic value when ERCP is unsuccessful.
ERCP cannot be performed after biliary-enteric an-astomosis when the anastomosis is beyond the duedonum, as is frequently the case. Likewise, ERCP cannot be performed after gastro-enterostomy such as a Billroth 2. MRCP may be preferred to ERCP in patients with sus-pected solid extraductal masses, or cystic masses that do not communicate with the duct system[21]. Patient preference for non-invasive imaging may also be a conside-ration to perform MRCP rather than ERCP. It is likely that in the near future MRCP will replace diagnostic ERCP as the modality of choice for imaging the biliary and pancreatic ducts.
S- Editor Liu Y L- Editor Alpini GD E- Editor Che YB
| 1. | Fulcher AS, Turner MA, Capps GW, Zfass AM, Baker KM. Half-Fourier RARE MR cholangiopancreatography: experience in 300 subjects. Radiology. 1998;207:21-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 215] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 2. | Guibaud L, Bret PM, Reinhold C, Atri M, Barkun AN. Bile duct obstruction and choledocholithiasis: diagnosis with MR cholangiography. Radiology. 1995;197:109-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 186] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 3. | Regan F, Fradin J, Khazan R, Bohlman M, Magnuson T. Choledocholithiasis: evaluation with MR cholangiography. AJR Am J Roentgenol. 1996;167:1441-1445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 90] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | Taourel P, Bret PM, Reinhold C, Barkun AN, Atri M. Anatomic variants of the biliary tree: diagnosis with MR cholangiopancreatography. Radiology. 1996;199:521-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 136] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 5. | Hirohashi S, Hirohashi R, Uchida H, Akira M, Itoh T, Haku E, Ohishi H. Pancreatitis: evaluation with MR cholangiopancreatography in children. Radiology. 1997;203:411-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 49] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Matos C, Metens T, Devière J, Nicaise N, Braudé P, Van Yperen G, Cremer M, Struyven J. Pancreatic duct: morphologic and functional evaluation with dynamic MR pancreatography after secretin stimulation. Radiology. 1997;203:435-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 250] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 7. | Barish MA, Soto JA. MR cholangiopancreatography: techniques and clinical applications. AJR Am J Roentgenol. 1997;169:1295-1303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 82] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Miyazaki T, Yamashita Y, Tsuchigame T, Yamamoto H, Urata J, Takahashi M. MR cholangiopancreatography using HASTE (half-Fourier acquisition single-shot turbo spin-echo) sequences. AJR Am J Roentgenol. 1996;166:1297-1303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 145] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 9. | Irie H, Honda H, Tajima T, Kuroiwa T, Yoshimitsu K, Makisumi K, Masuda K. Optimal MR cholangiopancreatographic sequence and its clinical application. Radiology. 1998;206:379-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 87] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 10. | Yamashita Y, Abe Y, Tang Y, Urata J, Sumi S, Takahashi M. In vitro and clinical studies of image acquisition in breath-hold MR cholangiopancreatography: single-shot projection technique versus multislice technique. AJR Am J Roentgenol. 1997;168:1449-1454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 57] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Fulcher AS, Turner MA, Capps GW. MR cholangiography: technical advances and clinical applications. Radiographics. 1999;19:25-41; discussion 41-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 64] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Fulcher AS, Turner MA. MR pancreatography: a useful tool for evaluating pancreatic disorders. Radiographics. 1999;19:5-24; discussion 41-44; quiz 148-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 83] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 13. | Lillemoe KD, Pitt HA, Cameron JL. Current management of benign bile duct strictures. Adv Surg. 1992;25:119-174. [PubMed] |
| 14. | Lee MG, Lee HJ, Kim MH, Kang EM, Kim YH, Lee SG, Kim PN, Ha HK, Auh YH. Extrahepatic biliary diseases: 3D MR cholangiopancreatography compared with endoscopic retrograde cholangiopancreatography. Radiology. 1997;202:663-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 106] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Khalid TR, Casillas VJ, Montalvo BM, Centeno R, Levi JU. Using MR cholangiopancreatography to evaluate iatrogenic bile duct injury. AJR Am J Roentgenol. 2001;177:1347-1352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 50] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Vitellas KM, El-Dieb A, Vaswani K, Bennett WF, Fromkes J, Steinberg S, Bova JG. Detection of bile duct leaks using MR cholangiography with mangfodipir trisodium (Teslascan). J Comput Assist Tomogr. 2001;25:102-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Bret PM, Reinhold C, Taourel P, Guibaud L, Atri M, Barkun AN. Pancreas divisum: evaluation with MR cholangiopancreatography. Radiology. 1996;199:99-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 160] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 18. | Soto JA, Barish MA, Yucel EK, Clarke P, Siegenberg D, Chuttani R, Ferrucci JT. Pancreatic duct: MR cholangiopancreatography with a three-dimensional fast spin-echo technique. Radiology. 1995;196:459-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 124] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 19. | Nealon WH, Townsend CM, Thompson JC. Preoperative endoscopic retrograde cholangiopancreatography (ERCP) in patients with pancreatic pseudocyst associated with resolving acute and chronic pancreatitis. Ann Surg. 1989;209:532-538; discussion 538-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 58] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Freeny PC, Marks WM, Ryan JA, Traverso LW. Pancreatic ductal adenocarcinoma: diagnosis and staging with dynamic CT. Radiology. 1988;166:125-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 189] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 21. | Schwartz LH, Coakley FV, Sun Y, Blumgart LH, Fong Y, Panicek DM. Neoplastic pancreaticobiliary duct obstruction: evaluation with breath-hold MR cholangiopancreatography. AJR Am J Roentgenol. 1998;170:1491-1495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 63] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Semelka RC, Kelekis NL, John G, Ascher SM, Burdeny D, Siegelman ES. Ampullary carcinoma: demonstration by current MR techniques. J Magn Reson Imaging. 1997;7:153-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Traverso LW, Peralta EA, Ryan JA, Kozarek RA. Intraductal neoplasms of the pancreas. Am J Surg. 1998;175:426-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 122] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 24. | Warshaw AL, Compton CC, Lewandrowski K, Cardenosa G, Mueller PR. Cystic tumors of the pancreas. New clinical, radiologic, and pathologic observations in 67 patients. Ann Surg. 1990;212:432-443; discussion 444-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 431] [Cited by in RCA: 387] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 25. | Fukukura Y, Fujiyoshi F, Sasaki M, Ichinari N, Inoue H, Kajiya Y, Nakajo M. HASTE MR cholangiopancreatography in the evaluation of intraductal papillary-mucinous tumors of the pancreas. J Comput Assist Tomogr. 1999;23:301-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Silas AM, Morrin MM, Raptopoulos V, Keogan MT. Intraductal papillary mucinous tumors of the pancreas. AJR Am J Roentgenol. 2001;176:179-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Bradley EL, Young PR, Chang MC, Allen JE, Baker CC, Meredith W, Reed L, Thomason M. Diagnosis and initial management of blunt pancreatic trauma: guidelines from a multiinstitutional review. Ann Surg. 1998;227:861-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 179] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 28. | Kozarek RA, Ball TJ, Patterson DJ, Freeny PC, Ryan JA, Traverso LW. Endoscopic transpapillary therapy for disrupted pancreatic duct and peripancreatic fluid collections. Gastroenterology. 1991;100:1362-1370. [PubMed] |
| 29. | Nirula R, Velmahos GC, Demetriades D. Magnetic resonance cholangiopancreatography in pancreatic trauma: a new diagnostic modality? J Trauma. 1999;47:585-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Soto JA, Alvarez O, Múnera F, Yepes NL, Sepúlveda ME, Pérez JM. Traumatic disruption of the pancreatic duct: diagnosis with MR pancreatography. AJR Am J Roentgenol. 2001;176:175-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 69] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 31. | Pavone P, Laghi A, Catalano C, Broglia L, Panebianco V, Messina A, Salvatori FM, Passariello R. MR cholangiography in the examination of patients with biliary-enteric anastomoses. AJR Am J Roentgenol. 1997;169:807-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 62] [Article Influence: 2.2] [Reference Citation Analysis (0)] |