Published online May 7, 2007. doi: 10.3748/wjg.v13.i17.2461
Revised: March 8, 2007
Accepted: March 12, 2007
Published online: May 7, 2007
Hepatitis C virus (HCV) infects approximately 170 million individuals worldwide. Prevention of HCV infection complications is based on antiviral therapy with the combination of pegylated interferon alfa and ribavirin. The use of serological and virological tests has become essential in the management of HCV infection in order to diagnose infection, guide treatment decisions and assess the virological response to antiviral therapy. Anti-HCV antibody testing and HCV RNA testing are used to diagnose acute and chronic hepatitis C. The HCV genotype should be systematically determined before treatment, as it determines the indication, the duration of treatment, the dose of ribavirin and the virological monitoring procedure. HCV RNA monitoring during therapy is used to tailor treatment duration in HCV genotype 1 infection, and molecular assays are used to assess the end-of-treatment and, most importantly the sustained virological response, i.e. the endpoint of therapy.
- Citation: Chevaliez S, Pawlotsky JM. Hepatitis C virus: Virology, diagnosis and management of antiviral therapy. World J Gastroenterol 2007; 13(17): 2461-2466
- URL: https://www.wjgnet.com/1007-9327/full/v13/i17/2461.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i17.2461
Hepatitis C virus (HCV) infects approximately 170 million individuals worldwide. Chronic HCV infection has been estimated to be responsible for approximately 250000 to 350000 deaths per year, essentially related to decompensation of cirrhosis, end-stage liver disease and hepatocellular carcinoma. Prevention of HCV infection complications can be achieved by antiviral therapy based on the use of a combination of pegylated interferon (IFN) alfa and ribavirin, that yields a sustained eradication of infection in 40% to 50% of cases[1]. The use of serological and virological tests has become essential in the management of HCV infection in order to diagnose infection, and most importantly guide treatment decisions and assess the virological response to antiviral therapy.
HCV is a member of the Flaviviridae family, genus Hepacivirus[2]. Six HCV genotypes[1-6] and a large number of subtypes (1a, 1b, 1c, etc.) have been identified so far[2]. HCV only natural host is man. All HCV genotypes have a common ancestor virus. However, HCV genotypes 1, 2, and 4 emerged and diversified in Central and Western Africa, genotype 5 in South Africa, and genotypes 3 and 6 in China, South-East Asia and the Indian subcontinent. In these areas, a large number of subtypes of these genotypes are found[2]. The rest of the world, in particular industrialized areas, harbor a small number of HCV subtypes that could widely spread because they met an efficient route of transmission, such as blood transfusion or the intravenous use of drugs. They include genotypes 1a, 1b, 2a, 2b, 2c, 3a, 4a and 5a[2].
The HCV virion is made of a single-stranded positive RNA genome, contained into an icosahedral capsid, itself enveloped by a lipid bilayer within which two different glycoproteins are anchored[3]. The genome contains three distinct regions: (1) a short 5′ non-coding region that contains two domains, a stem-loop structure involved in positive-strand priming during HCV replication and the internal ribosome entry site (IRES), the RNA structure responsible for attachment of the ribosome and polyprotein translation; (2) a long, unique open reading frame (ORF) of more than 9000 nucleotides which is translated into a precursor polyprotein, secondarily cleaved to give birth to the structural proteins (the capsid protein C and the two envelope glycoproteins E1 and E2) and to the non-structural proteins (p7, NS2, NS3, NS4A, NS4B, NS5A, and NS5B). The functions of the non-structural proteins have been elucidated by a large number of studies and by analogy to related viruses; only NS4B and NS5A have no well-defined functions to date; (3) a short 3′ non-coding region principally involved in minus-strand priming during HCV replication[3]. The 3' UTR has been divided into three regions: a variable sequence of approximately 40 bases, a variable length poly-UC rich tract, and a highly conserved 98 base region.
The HCV lifecycle starts with virion attachment to its specific receptor[3]. Several candidate molecules have been suggested to play a role in the receptor complex, including tetraspanin CD81, the scavenger receptor BI (SR-BI), the adhesion molecules DC-SIGN and L-SIGN and the low-density lipoprotein (LDL) receptor. The HCV lifecyle is poorly known because of the lack, until very recently, of a productive culture system. It is supposed, by analogy with the Flaviviridae, that the virus linked to its receptor complex is internalized, and that the nucleocapsid is released into the cytoplasm. The virus is decapsidated, and the genomic HCV RNA is used both for polyprotein translation and replication in the cytoplasm. Replication and post-translational processing appear to take place in a membranous web made of the non-structural proteins and host cell proteins called "replication complex", located in close contact with perinuclear membranes. Genome encapsidation appears to take place in the endoplasmic reticulum and nucleocapsids are enveloped and matured into the Golgi apparatus before newly produced virions are released in the pericellular space by exocytosis[3].
Anti-HCV antibody detection: The detection of anti-HCV antibodies in plasma or serum is based on the use of third-generation enzyme immunoassays (EIAs), that detect antibodies directed against various HCV epitopes. Recombinant antigens are used to capture circulating anti-HCV antibodies onto the wells of microtiter plates, microbeads, or specific holders adapted to closed auto-mated devices. The presence of anti-HCV antibodies is revealed by anti-antibodies labeled with an enzyme that catalyzes the transformation of a substrate into a colored compound. The optical density (OD) ratio of the reaction (sample OD/internal control OD) is proportional to the amount of antibodies in the serum or plasma sample[4]. The specificity of third-generation EIAs for anti-HCV is greater than 99%[5]. Their sensitivity is more difficult to determine, given the lack of a gold standard method, but it is excellent in HCV-infected immunocompetent patients. EIAs can be fully automated and are well adapted to large volume testing. Immunoblot tests are nowadays clinically obsolete given the good performance of third-generation anti-HCV EIAs[6].
Serological determination of the HCV genotype: The HCV genotype can be determined by seeking for antibodies directed to genotype-specific HCV epitopes with a competitive EIA. The currently available assay (Murex HCV serotyping 1-6 HC02, Abbott Laboratories, North Chicago, Illinois) identifies the type (1 to 6), but does not discriminate among the subtypes, and provides interpretable results in approximately 90% of chronically infected immunocompetent patients[7]. Mixed serological reactivities can be observed that could be related to mixed infection although cross-reactivity or recovery from one genotype infection and persistence of viremia with another genotype cannot be ruled out.
For many years, the HCV RNA quantitative units used in the various assays did not represent the same amount of HCV RNA in a clinical sample. The World Health Organization (WHO) has established an international standard for universal standardization of HCV RNA quantification units. An HCV RNA international unit (IU) has been defined, which is currently used in all of the commercial HCV RNA quantitative assays and should be preferred to any other quantitative unit. Indeed, the use of standardized IUs for HCV RNA quantification allows deriving recommendations and guidelines from clinical trials and applying them in clinical practice with any HCV RNA assay.
Qualitative, non-quantitative HCV RNA detection: Qualitative detection assays are based on the principle of target amplification using either "classic" polymerase chain reaction (PCR), "real-time" PCR or "transcription-mediated amplification"(TMA)[8]. HCV RNA is extracted and reverse transcribed into a single-stranded complementary DNA (cDNA), which is subsequently processed into a cyclic enzymatic reaction leading to the generation of a large number of detectable copies. Double-stranded DNA copies of HCV genome are synthesized in PCR-based assays, whereas single-stranded RNA copies are generated in TMA. Detection of amplified products is achieved by hybridizing the produced amplicons onto specific probes after the reaction in "classic" PCR or TMA techniques[8]. In "real-time" PCR, each round of amplification leads to the emission of a fluorescent signal and the number of signals per cycle is proportional to the amount of HCV RNA in the starting sample[8-10]. Qualitative detection assays must detect 50 HCV RNA IU/mL or less, and have equal sensitivity for the detection of all HCV genotypes. The lower limit of detection of the qualitative, nonquantitative reverse-transcriptase PCR-based assay Amplicor® HCV v2.0, or of its semi-automated version Cobas® Amplicor® HCV v2.0 (Roche Molecular Systems, Pleasanton, California) is 50 IU/mL, whereas that of the TMA-based assay Versant® HCV RNA Qualitative Assay (Siemens, Tarrytown, New York) is 10 IU/mL. Real-time PCR assays, which are also able to quantify HCV RNA, have lower limits of detection of 15 IU/mL (Cobas Ampliprep®-Cobas Taqman® (CAP-CTM) HCV Test, Roche Molecular Systems) and of 12-30 IU/mL according to the amount of blood tested (Abbott RealTimeTM HCV Assay, Abbott Diagnostic) when they are used as purely qualitative, nonquantitative assays.
HCV RNA quantification: HCV RNA can be quantified by means of target amplification techniques (competitive PCR or real-time PCR) or signal amplification techniques [branched DNA (bDNA) assay][8]. Five standardized assays are commercially available. Two of them are based on competitive PCR: Amplicor HCV Monitor® v2.0 and its semi-automated version Cobas® Amplicor HCV Monitor® v2.0 (Roche Molecular Systems), and LCx® HCV RNA Quantitative Assay (Abbott Laboratories); one is based on bDNA technology, Versant® HCV RNA 3.0 Assay (Siemens); and two are based on real-time PCR amplification, Cobas® TaqMan HCV Test, which can be coupled with automated extraction in Cobas Ampliprep® (CAP-CTM, Roche Molecular Systems), and Abbott RealTimeTM HCV assay (Abbott Diagnostics), which uses the Abbott m2000RT system and can also be coupled with an automated extraction procedure in m2000SP (m2000 Real-Time PCR System). HCV RNA levels falling above the upper limit of quantification of the assay are underestimated and the samples must be retested after 1/10 to 1/100 dilution in order to achieve accurate quantification. The Cobas® TaqMan HCV Test has been shown to underquantify some HCV genotype 4 and, less often, HCV genotype 2 samples[11]. In addition, differences in calibration of the assays relative to the primary WHO HCV RNA standard lead to slight differences between the results given in the same samples by different assays in spite of the use of international units as a quantification unit[11]. However, the most promising approach for the future is fully automated real-time PCR assays.
The reference method for HCV genotype determination is direct sequencing of the NS5B or E1 regions of HCV genome by means of "in-house" techniques, followed by sequence alignment with prototype sequences and phylogenetic analysis[2,12]. In clinical practice, HCV genotype can be determined by various commercial kits, using direct sequence analysis of the 5' noncoding region (Trugene® 5' NC HCV Genotyping Kit, Bayer HealthCare) or reverse hybridization analysis using genotype-specific probes located in the 5' noncoding region (commercialized as INNO-LiPA HCV II, Innogenetics, Ghent, Belgium, or Versant® HCV Genotyping Assay, Bayer HealthCare)[13-16]. Mistyping is rare with these techniques, but mis-subtyping may occur in 10% to 25% of cases, related to the studied region (5' noncoding region) rather than the technique used. These errors have no clinical consequences, because only the type is used for therapeutic decision-making. An assay based on direct sequencing of the NS5B region is currently in development (Trugene® NS5B HCV Genotyping Kit, Bayer HealthCare).
Patients with a suspicion of acute hepatitis C should be tested for both anti-HCV antibodies by EIA and HCV RNA with a sensitive technique, i.e. an HCV RNA assay with a lower limit of detection of 50 IU/mL or less[4]. Four marker profiles can be observed according to the presence or absence of either marker. The presence of HCV RNA in the absence of anti-HCV antibodies is strongly indicative of acute HCV infection, which will be confirmed by seroconversion (i.e. the appearance of anti-HCV antibodies) a few days to week later. Acutely infected patients can also have both HCV RNA and anti-HCV antibodies at the time of diagnosis. It is difficult, in this case, to distinguish acute hepatitis C from an acute exacerbation of chronic hepatitis C or an acute hepatitis of another cause in a patient with chronic hepatitis C.
Acute hepatitis C is very unlikely if both anti-HCV antibodies and HCV RNA are absent or if anti-HCV antibodies are present without HCV RNA. The latter patients should however be retested after a few week because HCV RNA can be temporarily undetectable, due to transient, partial control of viral replication before infection becomes chronic[17]. Apart from such cases, the presence of anti-HCV antibodies in the absence of HCV RNA is generally seen in patients who have recovered from a past HCV infection. Nevertheless, this pattern cannot be differentiated from a false positive EIA result, the exact prevalence of which is unknown.
In patients with clinical or biological signs of chronic liver disease, chronic hepatitis C is certain when both anti-HCV antibodies and HCV RNA are present[6,18]. Detectable HCV replication in the absence of anti-HCV antibodies is exceptional with the current enzyme immunoassays, almost exclusively observed in profoundly immunodepressed patients, hemodialysis patients or agammaglobulinemic subjects[19,20].
In patients who have no indication for therapy or have a contra-indication to the use of antiviral drugs, virological tests have no prognostic value. Thus, they cannot be used to predict the natural course of infection or the onset of extrahepatic manifestations. In untreated patients, the severity of liver inflammation and fibrosis must be evaluated every three to five years by means of a liver biopsy or non-invasive serological or ultrasound-based testing[1].
The current standard treatment for chronic hepatitis C is the combination of pegylated interferon (IFN) alfa and ribavirin[1]. The efficacy endpoint of hepatitis C treatment is the "sustained virological response" (SVR), defined by the absence of detectable HCV RNA in serum as assessed by an HCV RNA assay with a lower limit of detection of 50 IU/mL or less 24 wk after the end of treatment[1]. The advent of new, more sensitive assays with different lower limits of HCV RNA detection may create some confusion as to whether patients have "undetectable" HCV RNA during therapy.
Only patients with detectable HCV RNA should be considered for pegylated IFN alfa and ribavirin combination therapy[1]. The decision to treat patients with chronic hepatitis C depends on multiple parameters, including a precise assessment of the severity of liver disease and of its foreseeable outcome, the presence of absolute or relative contra-indications to therapy, and the patient's willingness to be treated.
The HCV genotype should be systematically deter-mined before treatment, as it determines the indication, the duration of treatment, the dose of ribavirin and the virological monitoring procedure[21].
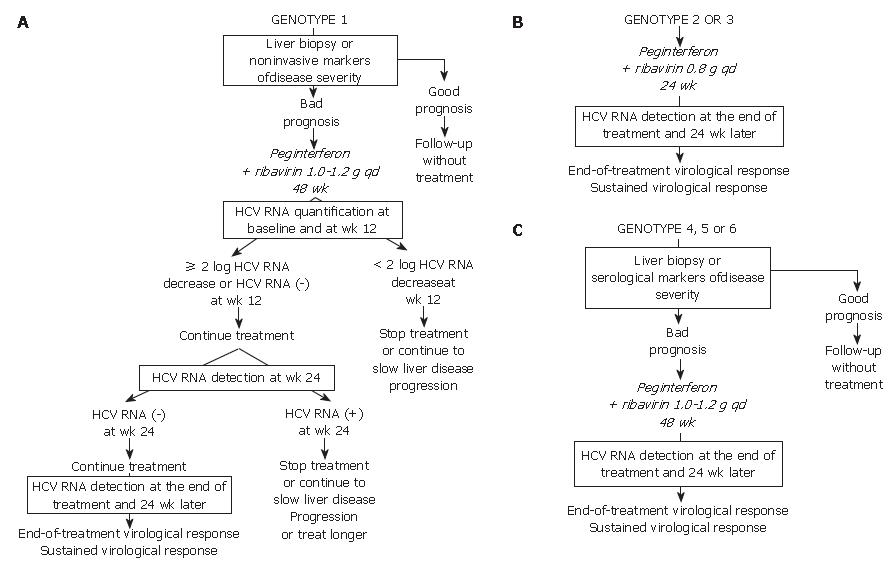
Given the likelihood of a sustained virological response, of the order of 40% to 50%, a precise assessment of liver disease prognosis by means of a liver biopsy or a non-invasive method based on serological markers of fibrosis or ultrasound-based testing[22,23] must be performed in order to help with the treatment decision (Figure 1A). It is recommended not to treat patients with mild lesions and to re-assess their liver disease after 3 to 5 years. The patients with inflammation and/or fibrosis (Metavir score A ≥ 2 and/or F ≥ 2) have an indication for therapy[1].
The approved dose of pegylated IFN alfa-2a is 180 μg per week, independent of body weight, whereas that of pegylated IFN alfa-2b is weight-adjusted at 1.5 μg/kg per week, identical for all HCV genotypes. Patients infected with HCV genotype 1 should receive a high dose of ribavirin, i.e. 1000 to 1200 mg daily, based on body weight less than or greater than 75 kg. The heaviest patients could benefit from a higher ribavirin dose, up to 1600 mg daily. Genotype 1-infected patients theoretically require 48 wk of treatment (Figure 1A)[1]. Monitoring of HCV RNA load decrease during therapy must be performed in order to avoid treating patients with no likelihood of a subsequent SVR[24,25]. For this purpose, HCV RNA quantification must be performed at baseline and after 12 wk of treatment (Figure 1A) with the same technique in order to ensure comparability of the results at the two time points[1]. Treatment must be continued when there is a 2-log drop in HCV RNA level, i.e. when baseline HCV RNA level is divided by 100 or more, or when HCV RNA is undetectable at wk 12[1]. In these patients, it is recommended to assess the presence of HCV RNA with a sensitive technique (lower limit of detection: 50 IU/mL or less) at wk 24. If HCV RNA is undetectable at wk 24, treatment must be continued until wk 48, with a high likelihood of an SVR. It was recently suggested that 24 wk of therapy might be sufficient for patients with a low baseline viral load, a 2-log or more decline at wk 12 and undetectable HCV RNA at wk 24[26]. Rapid virological responders, defined as patients with no detectable HCV RNA at wk 4 of therapy could also benefit from shorter therapy. Ongoing trials will feed future guidelines.
In contrast, if HCV RNA is still detectable at wk 24, the likelihood of an SVR is virtually nil and treatment can be stopped or continued with the only aim to slow liver disease progression in patients with a severe prognosis, without any hope to eradicate infection (Figure 1A)[1,24]. Ongoing trials are studying whether a prolonged antiviral treatment or maintenance therapy with pegylated IFN alfa monotherapy could be beneficial in these patients. When treatment is continued until wk 48, the end-of-treatment and sustained virological responses should be assessed by means of a sensitive HCV RNA assay, with a lower limit of detection of 50 IU/mL or less[1]. HCV RNA detection at the end of therapy is highly predictive of a post-treatment relapse (the more sensitive assays allow earlier identification of relapser patients), whereas the absence of HCV RNA at the end of treatment indicates a virological response. The latter patients must be retested for HCV RNA with a sensitive method 24 wk later in order to assess the SVR, i.e. the endpoint of therapy[1,4]. HCV infection appears to be definitively cured in the vast majority of sustained virological responders.
The lack of a 12-wk virological response (no change or an HCV RNA decrease of less than 2 logs at wk 12) is associated with a virtually nil probability of a subsequent sustained virological response[24,25]. Treatment can thus be stopped at wk 12 in these patients, or continued to slow liver disease progression without clearing the virus (Figure 1A). The benefits of maintenance therapy on the outcome of HCV-associated liver disease are currently under investi-gation. This "stopping rule", based on monitoring of HCV RNA load reduction at wk 12, was recently shown to also apply to patients co-infected with HCV and human immunodeficiency virus[27-29].
Patients infected with HCV genotypes 2 or 3 have a 70%-80% likelihood of an SVR with a low dose of ribavirin and only 24 wk of treatment[21,25,30]. Thus, in the absence of contra-indications, these patients should be treated regardless of the severity of their liver disease (Figure 1B). The recommended dose of pegylated IFN alfa-2a or alfa-2b is the same as for HCV genotype 1. The fixed recommended dose of ribavirin is 800 mg per day (Figure 1B)[1]. It is possible that lower doses of ribavirin and/or shorter duration of treatment could be sufficient to achieve an SVR in certain subgroups of patients with genotype 2 or 3 infection, such as those with a low baseline viral load and no extensive fibrosis or cirrhosis, as suggested by recent preliminary data[31]. One should be careful in patients who combine several baseline parameters of non-response, such as extensive fibrosis, an old age and a male gender, who might need 48 wk of therapy to clear infection.
No monitoring of HCV RNA level changes during therapy is recommended in the patients with genotype 2 or 3 infection, because the vast majority of them become HCV RNA-negative early during treatment. Like in HCV genotype 1-infected patients, the virological response must be assessed by means of a sensitive HCV RNA assay at the end of therapy and 24 wk later in order to determine whether the virological response is sustained (Figure 1B)[1,4].
In the absence of any clinical trial including a sufficient number of patients, the likelihood of an SVR and the optimal treatment schedule remain unknown for the patients infected with HCV genotypes 4, 5 or 6. It is thus recommended to treat them like those infected with HCV genotype 1, i.e. with pegylated IFN alfa at the usual dose, combined with a high dose of ribavirin (1000-1200 mg per day, according to body weight less or greater than 75 kg) (Figure 1C). In the absence of published data, no stopping rules have been defined and it is recommended to treat these patients for a total of 48 wk. The virological response must be assessed by means of a sensitive HCV RNA assay (lower limit of detection of 50 IU/mL or less) at the end of therapy and 24 wk later[1,4].
Virological tools have nowadays become mandatory at every step of HCV infection treatment. Algorithms have been derived that allow the clinician to tailor treatment schedules to the individual patient and his/her virological response to therapy, in order to optimize the results of pegylated IFN-ribavirin therapy. This approach is cost-effective, because treatment dose and duration are adapted to the patient's needs and administration can be stopped when the likelihood of a sustained virological response is nil.
In the future, more sensitive HCV RNA assays will be available. They will better differentiate responder patients who will subsequently relapse from those who will not. They will also detect the virological relapse earlier after the end of therapy. A number of ongoing studies are currently assessing the capacity of viral load measurements at earlier time points to predict the sustained virological response or non-response. New algorithms will be developed soon, based on the assessment of viral load reductions at wk 4 of therapy, or eventually earlier. Too complicated algorithms applied to a too high number of patient subgroups may however make therapy more and more difficult on an every day basis. In addition, early predictions based on frequent viral load measurements may not always be easily feasible in the clinical setting. Overall, it will be important to keep treatment algorithms as simple and feasible as possible in order to offer the best chance of success to the individual patients.
S- Editor Wang J E- Editor Liu Y
| 1. | NIH Consensus Statement on Management of Hepatitis C: 2002. NIH Consens State Sci Statements. 2002;19:1-46. [PubMed] |
| 2. | Simmonds P, Bukh J, Combet C, Deléage G, Enomoto N, Feinstone S, Halfon P, Inchauspé G, Kuiken C, Maertens G. Consensus proposals for a unified system of nomenclature of hepatitis C virus genotypes. Hepatology. 2005;42:962-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1070] [Cited by in RCA: 1083] [Article Influence: 54.2] [Reference Citation Analysis (0)] |
| 3. | Penin F, Dubuisson J, Rey FA, Moradpour D, Pawlotsky JM. Structural biology of hepatitis C virus. Hepatology. 2004;39:5-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 458] [Cited by in RCA: 458] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 4. | Pawlotsky JM. Use and interpretation of virological tests for hepatitis C. Hepatology. 2002;36:S65-S73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 97] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 5. | Colin C, Lanoir D, Touzet S, Meyaud-Kraemer L, Bailly F, Trepo C. Sensitivity and specificity of third-generation hepatitis C virus antibody detection assays: an analysis of the literature. J Viral Hepat. 2001;8:87-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 151] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 6. | Pawlotsky JM, Lonjon I, Hezode C, Raynard B, Darthuy F, Remire J, Soussy CJ, Dhumeaux D. What strategy should be used for diagnosis of hepatitis C virus infection in clinical laboratories? Hepatology. 1998;27:1700-1702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 93] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Pawlotsky JM, Prescott L, Simmonds P, Pellet C, Laurent-Puig P, Labonne C, Darthuy F, Remire J, Duval J, Buffet C. Serological determination of hepatitis C virus genotype: comparison with a standardized genotyping assay. J Clin Microbiol. 1997;35:1734-1739. [PubMed] |
| 8. | Pawlotsky JM. Molecular diagnosis of viral hepatitis. Gastroenterology. 2002;122:1554-1568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 150] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 9. | Martell M, Gómez J, Esteban JI, Sauleda S, Quer J, Cabot B, Esteban R, Guardia J. High-throughput real-time reverse transcription-PCR quantitation of hepatitis C virus RNA. J Clin Microbiol. 1999;37:327-332. [PubMed] |
| 10. | Komurian-Pradel F, Paranhos-Baccalà G, Sodoyer M, Chevallier P, Mandrand B, Lotteau V, André P. Quantitation of HCV RNA using real-time PCR and fluorimetry. J Virol Methods. 2001;95:111-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 78] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Chevaliez S, Bouvier-Alias M, Brillet R, Pawlotsky JM. Overestimation and underestimation of hepatitis C virus RNA levels in a widely used real-time polymerase chain reaction-based method. Hepatology. 2007;46:22-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 108] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 12. | Simmonds P. Viral heterogeneity of the hepatitis C virus. J Hepatol. 1999;31 Suppl 1:54-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 155] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 13. | Germer JJ, Vandenameele JN, Mitchell PS, Harmsen WS, Yao JD. Automated sample preparation for the TRUGENE HIV-1 genotyping kit using the MagNA pure LC instrument. Diagn Microbiol Infect Dis. 2004;49:59-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Stuyver L, Wyseur A, van Arnhem W, Hernandez F, Maertens G. Second-generation line probe assay for hepatitis C virus genotyping. J Clin Microbiol. 1996;34:2259-2266. [PubMed] |
| 15. | Stuyver L, Wyseur A, van Arnhem W, Lunel F, Laurent-Puig P, Pawlotsky JM, Kleter B, Bassit L, Nkengasong J, van Doorn LJ. Hepatitis C virus genotyping by means of 5'-UR/core line probe assays and molecular analysis of untypeable samples. Virus Res. 1995;38:137-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 113] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 16. | Zheng X, Pang M, Chan A, Roberto A, Warner D, Yen-Lieberman B. Direct comparison of hepatitis C virus genotypes tested by INNO-LiPA HCV II and TRUGENE HCV genotyping methods. J Clin Virol. 2003;28:214-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Lavillette D, Morice Y, Germanidis G, Donot P, Soulier A, Pagkalos E, Sakellariou G, Intrator L, Bartosch B, Pawlotsky JM. Human serum facilitates hepatitis C virus infection, and neutralizing responses inversely correlate with viral replication kinetics at the acute phase of hepatitis C virus infection. J Virol. 2005;79:6023-6034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 214] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 18. | EASL International Consensus Conference on Hepatitis C. Paris, 26-28, February 1999, Consensus Statement. European Association for the Study of the Liver. J Hepatol. 1999;30:956-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 445] [Cited by in RCA: 410] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 19. | Lok AS, Chien D, Choo QL, Chan TM, Chiu EK, Cheng IK, Houghton M, Kuo G. Antibody response to core, envelope and nonstructural hepatitis C virus antigens: comparison of immunocompetent and immunosuppressed patients. Hepatology. 1993;18:497-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 78] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Thio CL, Nolt KR, Astemborski J, Vlahov D, Nelson KE, Thomas DL. Screening for hepatitis C virus in human immunodeficiency virus-infected individuals. J Clin Microbiol. 2000;38:575-577. [PubMed] |
| 21. | Hadziyannis SJ, Sette H, Morgan TR, Balan V, Diago M, Marcellin P, Ramadori G, Bodenheimer H, Bernstein D, Rizzetto M. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med. 2004;140:346-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2216] [Cited by in RCA: 2109] [Article Influence: 100.4] [Reference Citation Analysis (0)] |
| 22. | Castéra L, Vergniol J, Foucher J, Le Bail B, Chanteloup E, Haaser M, Darriet M, Couzigou P, De Lédinghen V. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1796] [Cited by in RCA: 1848] [Article Influence: 92.4] [Reference Citation Analysis (0)] |
| 23. | Poynard T, Imbert-Bismut F, Munteanu M, Ratziu V. FibroTest-FibroSURE: towards a universal biomarker of liver fibrosis? Expert Rev Mol Diagn. 2005;5:15-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 24. | Davis GL, Wong JB, McHutchison JG, Manns MP, Harvey J, Albrecht J. Early virologic response to treatment with peginterferon alfa-2b plus ribavirin in patients with chronic hepatitis C. Hepatology. 2003;38:645-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 596] [Cited by in RCA: 575] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 25. | Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Gonçales FL, Häussinger D, Diago M, Carosi G, Dhumeaux D. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4847] [Cited by in RCA: 4747] [Article Influence: 206.4] [Reference Citation Analysis (0)] |
| 26. | Zeuzem S, Buti M, Ferenci P, Sperl J, Horsmans Y, Cianciara J, Ibranyi E, Weiland O, Noviello S, Brass C. Efficacy of 24 weeks treatment with peginterferon alfa-2b plus ribavirin in patients with chronic hepatitis C infected with genotype 1 and low pretreatment viremia. J Hepatol. 2006;44:97-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 364] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 27. | Chung RT, Andersen J, Volberding P, Robbins GK, Liu T, Sherman KE, Peters MG, Koziel MJ, Bhan AK, Alston B. Peginterferon Alfa-2a plus ribavirin versus interferon alfa-2a plus ribavirin for chronic hepatitis C in HIV-coinfected persons. N Engl J Med. 2004;351:451-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 716] [Cited by in RCA: 686] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 28. | Pawlotsky JM. Treating hepatitis C in "difficult-to-treat" patients. N Engl J Med. 2004;351:422-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | Torriani FJ, Rodriguez-Torres M, Rockstroh JK, Lissen E, Gonzalez-García J, Lazzarin A, Carosi G, Sasadeusz J, Katlama C, Montaner J. Peginterferon Alfa-2a plus ribavirin for chronic hepatitis C virus infection in HIV-infected patients. N Engl J Med. 2004;351:438-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 992] [Cited by in RCA: 955] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 30. | Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, Koury K, Ling M, Albrecht JK. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4736] [Cited by in RCA: 4558] [Article Influence: 189.9] [Reference Citation Analysis (0)] |
| 31. | Dalgard O, Bjøro K, Hellum KB, Myrvang B, Ritland S, Skaug K, Raknerud N, Bell H. Treatment with pegylated interferon and ribavarin in HCV infection with genotype 2 or 3 for 14 weeks: a pilot study. Hepatology. 2004;40:1260-1265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 247] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 32. | Pawlotsky JM. Therapy of hepatitis C: from empiricism to eradication. Hepatology. 2006;43:S207-S220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 132] [Article Influence: 6.9] [Reference Citation Analysis (0)] |