Published online Apr 28, 2007. doi: 10.3748/wjg.v13.i16.2328
Revised: March 3, 2006
Accepted: March 8, 2007
Published online: April 28, 2007
AIM: To evaluate the efficacy of adalimumab induction therapy in patients with ulcerative colitis who previously responded to infliximab and then lost response or became intolerant.
METHODS: Ten patients with ulcerative colitis were enrolled in a 4-wk open-label trial. The patients received a loading dose of 160 mg adalimumab at wk 0 followed by 80 mg at wk 2. The primary efficacy measure was clinical improvement at wk 4, as defined by a decrease in clinical activity index (CAI) of more than 4.
RESULTS: Four of 10 patients (40%) benefited from subsequent adalimumab therapy; one patient achieved remission (CAI < 4) and 3 had clinical improvement at wk 4. 6 patients had no response (60%); 2 of 6 (33.3%) subsequently underwent colectomy. This was accompanied by a decrease in median CRP concentration from 16.8 mg/mL at baseline to 3.85 mg/mL at wk 4, excluding two patients who underwent colectomy after two infusions of adalimumab. Among the 6 patients with severe colitis (CAI > 12) at baseline, none achieved remission and only one patient had clinical improvement at wk 4.
CONCLUSION: The small advantage of adalimumab in patients with mild to moderate ulcerative colitis and lost response or intolerance to infliximab needs to be confirmed in randomised, double-blind, placebo-controlled trials.
- Citation: Peyrin-Biroulet L, Laclotte C, Roblin X, Bigard MA. Adalimumab induction therapy for ulcerative colitis with intolerance or lost response to infliximab: An open-label study. World J Gastroenterol 2007; 13(16): 2328-2332
- URL: https://www.wjgnet.com/1007-9327/full/v13/i16/2328.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i16.2328
Tumor necrosis factor (TNF) is a key proinflammatory cytokine in the pathogenesis of inflammatory bowel disease[1]. Although the implication of TNF in ulcerative colitis remains debated, this cytokine is found in increased concentrations in the blood, colonic tissue, and stools of patients with ulcerative colitis[2-4]. In addition, infliximab, a chimeric monoclonal antibody to TNF, has shown efficacy in inducing and maintaining response and remission in patients with moderate to severe ulcerative colitis in two large randomised placebo-controlled trials[5]. However, infliximab is immunogenic and repeated administration can result in the development of antibodies to infliximab that lead to infusion reactions, loss of efficacy, and delayed hypersensitivity reactions[6-8].
The development of an attenuated response or intolerance over time is a significant problem in inflam-matory bowel disease patients treated with infliximab therapy. Two anti-TNF agents which might be less immunogenic[9], namely adalimumab and certolizumab (a PEGylated humanized Fab’ fragment to TNF), have proven efficacy in the treatment of Crohn’s disease refractory to standard medical therapy with corticosteroids or immunomodulatory agents[10-15].
Adalimumab is a recombinant human IgG1 human monoclonal antibody that binds with high affinity and specificity to human soluble TNF but not to lymphotoxin. Recently, four uncontrolled trials[16-19] and a 4-wk rando-mised placebo-controlled trial named GAIN (Gauging Adalimumab Efficacy in Infliximab Non-Responders)[20] have demonstrated that adalimumab therapy was effective in inducing and maintaining remission in patients with active Crohn’s disease who had previously responded to infliximab and then lost response or became intolerant. The efficacy of adalimumab in patients with ulcerative colitis who had primary failure to infliximab remains unknown.
We conducted a 4-wk open-label trial to assess the efficacy of subcutaneous administration of adalimumab as induction therapy in patients with ulcerative colitis who had an attenuated response to infliximab or had become intolerant to infliximab therapy.
Eligible patients were males or females who were at least 18 years of age. Patients with ulcerative colitis who had previously responded to infliximab and then lost response or became intolerant were prospectively included. Loss of response to infliximab was defined as the presence of symptoms or evidence of disease activity as judged by their treating physician despite an increase of infliximab dosing to 10 mg/kg or a decrease in interval to 4 wk between infliximab infusions. Patients were classified as intolerant to infliximab if they had a history of discontinuation of infliximab as a result of a significant acute (reaction occurring during or within 24 h of an infliximab infusion) or delayed (reaction occurring more than 24 h after an infliximab infusion) infusion reaction. The diagnosis of ulcerative colitis was made at least 3 mo earlier using radiological, endoscopical, or histological evidence. Extent of disease was defined as left-sided if the disease was distal to the splenic flexure and as extensive if the disease extended proximal to the splenic flexure. The disease activity was assessed using the ulcerative colitis clinical activity index (CAI). This index incorporates eight items: stool frequency, blood in stools, general well being, abdominal pain, fever, extra-intestinal manifestations, sedimentation rate and haemoglobin[21]. Scores range from 0-32. A score of more than 12 on this index represents severe disease. A total score of 4-12 represents mild to moderate activity of colitis. A score of less than 4 represents quiescent ulcerative colitis.
Patients were ineligible if they had a serious infection, history of previously untreated tuberculosis or concurrent tuberculosis, previous history of demyelinating disease or malignancy (other than basal cell carcinoma of the skin), bowel resection within 4 wk of starting the study medication, symptomatic obstructive strictures, colostomy or ileostomy, history of drug or alcohol abuse, or poorly controlled medical conditions. Pregnant and lactating women were also excluded. All patients had a negative PPD skin test prior to starting adalimumab.
All patients gave consent and the protocol was approved by the Institutional Review Board at each center.
Concurrent therapies, including stable doses of 5-aminosalicylates, corticosteroids, azathioprine, 6-mercaptopurine, and methotrexate were permitted. All these agents were continued at a stable dose through wk 4.
This open-label study was performed at two centers in France (University Hospitals of Nancy and Grenoble). The pharmacist prepared 1.0 mL vials containing 40 mg/0.8 mL adalimumab. Injections of adalimumab were administered subcutaneously in the abdomen by medical staff at an on-site facility, and patients were monitored for 2 h following administration for adverse reactions. Given the results of the CLASSIC-I trial showing that only adalimumab 160/80 mg is superior to placebo for induction of remission in patients’ moderate to severe Crohn’s disease[10], all patients who were eligible received at wk 0 a loading dose of 160 mg adalimumab subcutaneously followed by an 80-mg dose at wk 2. Patients were followed through wk 4.
Patients were assessed at wk 0 and 4. At each visit, the CAI score was calculated, and blood samples were taken for measurement of plasma concentrations of C-reactive protein (CRP), as well as for haematological, biochemical and liver function assessments.
The primary outcome measure of the trial was the presence of clinical improvement by wk 4, as defined by decrease in the CAI score of more than 4. The proportion of patients who achieved remission to therapy at wk 4 as measured by a CAI score of less than 4, and changes in median CRP concentrations were examined as secondary outcome measures. Information regarding the Inflammatory Bowel Disease Questionnaire (IBDQ) and antibody to adalimumab was not collected.
The intention to treat (ITT) population included all patients who received at least one dose of study treatment. All patients were included in the safety analysis. Due to small sample size and the lack of placebo controls, statistical analysis was limited to descriptive statistics.
Between April 2006 and October 2006, a total of 10 adult patients were screened and were treated with adalimumab. Thus, the ITT population consists of 10 patients. The baseline characteristics of the patients are shown in Table 1. Eight adult males (80%) were included in this study. The median age of our population was 39 (range, 24-61) years, and the median duration of disease was 4.5 (range, 3-11) years at baseline. Seven patients (70%) had extensive disease. Of the patients, 6 patients (60%) had severe ulcerative colitis at the time of adalimumab therapy, and 4 (30%) had mild to moderate disease. The median CRP concentration was 16.8 mg/L at baseline. All patients had been previously treated with corticosteroids and azathioprine. The median number of previous infliximab infusions was 8.2 (range, 6-13). Seven patients (70%) were receiving at least one immunomodulator at the time of adalimumab therapy; 6 patients were on concurrent azathioprine, and one patient was on concurrent subcutaneous methotrexate. Six of the 10 patients (60%) experienced a loss of response to infliximab that led to discontinuation of such treatment, the remaining 4 patients became intolerant to infliximab therapy.
| Patient No. | Age (yr) | Sex(M/F) | Diseaseduration (yr) | Diseaseextent | Currentsomoker | Indication ofadalimumab | Infliximabinfusions (n) | Prior medications | Diseaseseverity | CRPconcentration (mg/L) | Concurrentmedications |
| 1 | 60 | M | 3 | E | No | LR | 6 | 5-ASA, steroid, AZA | Severe | 12.0 | None |
| 2 | 57 | M | 7 | LS | No | I | 7 | 5-ASA, steroid, AZA | Mild-moderate | 0.8 | AZA |
| 3 | 29 | F | 8 | LS | Yes | I | 9 | Steroid, AZA | Mild-moderate | 19.3 | AZA |
| 4 | 42 | M | 3 | E | No | LR | 8 | 5-ASA, steroid, AZA | Mild-moderate | 14.3 | None |
| 5 | 24 | M | 4 | E | No | LR | 13 | Steroid, AZA, MTX | Mild-moderate | 10.7 | MTX |
| 6 | 29 | F | 3 | E | No | LR | 7 | 5-ASA, Steroid, AZA, MTX | Severe | 14.2 | 5-ASA |
| 7 | 36 | M | 11 | LS | No | I | 6 | Steroid, AZA, MTX | Severe | 32.0 | AZA |
| 8 | 61 | M | 5 | E | No | LR | 10 | Steroid, AZA, MTX, CSA | Severe | 55.0 | AZA |
| 9 | 25 | M | 5 | E | No | I | 7 | Steroid, AZA, MTX, CSA | Severe | 40.0 | AZA |
| 10 | 45 | M | 4 | E | No | LR | 9 | Steroid, AZA | Severe | 24.0 | AZA |
All patients received two infusions of adalimumab and were followed through wk 4. All patients were able to tolerate adalimumab, including 4 who previously experienced acute or delayed hypersensitivity reactions with infliximab. One patient achieved remission (10%) after adalimumab treatment, 3 patients (30%) had clinical improvement, and 6 patients (60%) had no response (Table 2). Two of the 6 non-responders subsequently underwent total coloproctectomy after wk 2.
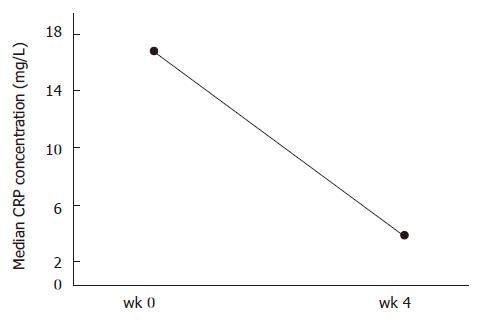
The median CRP value fell rapidly within the first month from 16.8 mg/L (range, 0.8-55) on study entry to 3.85 mg/L (range, 0.7-26) at wk 4, excluding two patients who underwent colectomy after two infusions of adalimumab (Figure 1).
The following baseline characteristics were broadly similar between patients who responded to therapy and those who had no response: age, disease duration, number of previous infliximab infusions, and concurrent medications. Notably, the percentage of subjects receiving concomitant azathioprine or methotrexate was similar at baseline (7 of 10, 70%) and in patients who had response at wk 4 (3 of 4, 75%).
Interestingly, response rate was lower in patients with extensive disease compared to left-sided ulcerative colitis (16.6% vs 50%, respectively). Two of 4 (50%) patients with prior intolerance to infliximab had clinical improvement or complete response (remission) at wk 4, while 2 of 6 (33.3%) patients with prior loss of response to infliximab had clinical improvement at wk 4. Among the 6 patients with severe ulcerative colitis as defined by CAI score > 12, adalimumab therapy resulted in remission in none of the patients, clinical improvement in only one patient (16.6%), and no response in the 5 remaining patients (83.3%). Baseline plasma CRP concentration was lower in responders than in nonresponders (median 16.8 mg/L, range 0.8-24 vs median 23.1 mg/L, range 10.7-55, respectively).
Adverse events were reported for two patients (20%), but none of these events led to patient withdrawal. One patient developed fungal dermatitis treated with specific medication and with a favourable outcome, and one patient experienced mild cutaneous rash of spontaneous favourable outcome. Both were judged as possibly drug-related adverse events by the investigators. None of the patients experienced serious adverse events. There were no clinically significant changes in laboratory values (haematological, biochemical and liver function tests) during the study.
Recently, a large Phase III, double-blind, placebo-controlled trial, named GAIN, showed that adalimumab was more effective than placebo for induction of remission in patients with moderate to severe Crohn’s disease who had primary failure to infliximab therapy[20].
In contrast, no data were available on the efficacy of adalimumab therapy in patients with ulcerative colitis and lost response or intolerance to infliximab. Our results suggest that adalimumab may be effective as induction therapy in some patients with ulcerative colitis who previously responded to infliximab and then became intolerant or lost response. In our study, 40% of the patients benefited from subsequent adalimumab therapy. The clinical efficacy of adalimumab was accompanied by a decrease in plasma CRP concentrations at wk 4, illustrating the potential benefit of adalimumab use in patients with ulcerative colitis who have primary failure to infliximab therapy. However, these results are counterbalanced by the low remission rate (10%) and the high rate of nonresponders (60%). In addition, these data should be interpreted with caution due to small sample size and the lack of placebo controls.
Patients who develop antibodies to biologic agents have a greater likelihood of acute and delayed infusion reactions, and a higher likelihood that long-term therapy will be compromised by eventual loss of response to an individual agent[6-8]. In our study, loss of response or intolerance to infliximab may have been regained by the introduction of a different biologic agent, such as adalimumab.
Patients with severe ulcerative colitis (i.e., high CAI score, high plasma CRP concentration, and/or extensive colitis) may be less likely to respond to adalimumab therapy, compared to those with mild or moderate disease. Furthermore, the percentage of patients who had clinical response at wk 4 was higher in patients with prior intolerance to infliximab (50%) than in patients with prior loss of response to infliximab (33.3%). These results indicate that subsequent adalimumab therapy might be more efficacious for patients who became intolerant to infliximab than for those with prior loss of response to infliximab. Finally, the percentage of subjects receiving concomitant azathioprine or methotrexate was similar at baseline (70%) and in patients who had clinical improvement or remission at wk 4 (75%). Thus, the concomitant immunosuppressive therapy does not seem to influence the clinical efficacy of adalimumab in this population. However, our study was underpowered to examine factors that predict patient response.
One question remains open: does adalimumab induction therapy reduce surgeries in this population? It should be noted that in this study, two patients underwent surgery before the end of the study, after receiving two infusions of adalimumab at wk 0 and 2. Moreover, two of the 6 nonresponders (patients 7 and 9) underwent colectomy after the end of the study, in November 2006 and December 2006, respectively. A longer duration of follow-up may thus be required to assess the long-term efficacy of subsequent adalimumab therapy in patients with loss of response or intolerance to infliximab. Furthermore, we did not analyse changes from baseline in IBDQ scores. Finally, efficacy of adalimumab in obtaining steroid-free remission could not be evaluated because none of the patients were treated with concurrent corticosteroids at baseline.
Importantly, none of the patients experienced into-lerance to adalimumab that led to discontinuation of the drug, including 4 who previously experienced acute or delayed hypersensitivity reactions with infliximab. Adalimumab therapy was generally well tolerated. Two patients developed cutaneous complications of favourable outcome. None of these adverse events led to patient withdrawal. No patients developed serious infections, malignancies, demyelinating disorders, drug-induced lupus, or died. However, this study was underpowered to detect these rare events. Trials of longer duration are required to evaluate the safety profile of adalimumab therapy in this population. Immunogenicity, i.e., occurrence of anti-adalimumab antibodies, was not evaluated in this study.
In conclusion, these results do not support the widespread use of adalimumab in the management of ulcerative colitis with intolerance or lost response to infliximab. A randomized controlled trial is necessary to further investigate the efficacy of adalimumab in patients with ulcerative colitis who had primary failure to infliximab.
S- Editor Wang J L- Editor Alpini GD E- Editor Liu Y
| 2. | Murch SH, Lamkin VA, Savage MO, Walker-Smith JA, MacDonald TT. Serum concentrations of tumour necrosis factor alpha in childhood chronic inflammatory bowel disease. Gut. 1991;32:913-917. |
| 3. | Murch SH, Braegger CP, Walker-Smith JA, MacDonald TT. Location of tumour necrosis factor alpha by immunohistochemistry in chronic inflammatory bowel disease. Gut. 1993;34:1705-1709. |
| 4. | Braegger CP, Nicholls S, Murch SH, Stephens S, MacDonald TT. Tumour necrosis factor alpha in stool as a marker of intestinal inflammation. Lancet. 1992;339:89-91. |
| 5. | Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, Travers S, Rachmilewitz D, Hanauer SB, Lichtenstein GR. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462-2476. |
| 6. | Farrell RJ, Alsahli M, Jeen YT, Falchuk KR, Peppercorn MA, Michetti P. Intravenous hydrocortisone premedication reduces antibodies to infliximab in Crohn's disease: a randomized controlled trial. Gastroenterology. 2003;124:917-924. |
| 7. | Baert F, Noman M, Vermeire S, Van Assche G, D' Haens G, Carbonez A, Rutgeerts P. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn's disease. N Engl J Med. 2003;348:601-608. |
| 8. | Hanauer SB, Wagner CL, Bala M, Mayer L, Travers S, Diamond RH, Olson A, Bao W, Rutgeerts P. Incidence and importance of antibody responses to infliximab after maintenance or episodic treatment in Crohn's disease. Clin Gastroenterol Hepatol. 2004;2:542-553. |
| 10. | Hanauer SB, Sandborn WJ, Rutgeerts P, Fedorak RN, Lukas M, MacIntosh D, Panaccione R, Wolf D, Pollack P. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn's disease: the CLASSIC-I trial. Gastroenterology. 2006;130:323-333; quiz 591. |
| 11. | Sandborn WJ, Hanauer SB, Rutgeerts P, Fedorak RN, Lukas M, Macintosh DG, Panaccione R, Wolf D, Kent JD, Bittle B. Adalimumab for maintenance treatment of Crohn's disease: results of the CLASSIC-II trial. Gut. 2007;56:1232-1239. |
| 12. | Colombel JF, Sandborn WJ, Rutgeerts P, Enns R, Hanauer SB, Panaccione R, Schreiber S, Byczkowski D, Li J, Kent JD. Adalimumab for maintenance of clinical response and remission in patients with Crohn's disease: the CHARM trial. Gastroenterology. 2007;132:52-65. |
| 13. | Schreiber S, Rutgeerts P, Fedorak RN, Khaliq-Kareemi M, Kamm MA, Boivin M, Bernstein CN, Staun M, Thomsen OØ, Innes A. A randomized, placebo-controlled trial of certolizumab pegol (CDP870) for treatment of Crohn's disease. Gastroenterology. 2005;129:807-818. |
| 14. | Sandborn WJ, Feagan BG, Stoinov S, Honiball PJ, Rutgeerts P, McColm JA, Innes A, Schreiber S. Certolizumab pegol administered subcutaneously is effective and well tolerated in patients with active Crohn's disease : results from a 26-week, placebo-controlled phase III study (PRECISE 1). Gastroenterology. 2006;130 Suppl 2:A107. |
| 15. | Schreiber S, Khalid-Kareemi M, Lawrance I, Hanauer S, McColm J, Bloomfield R, Sandborn W. Certolizumab pegol, a humanised anti-TNF pegylated Fab' fragment, is safe and effective in the maintenance of response and remission following induction in active Crohn's disease : a phase III study (PRECISE). Gut. 2005;54 Suppl:A82. |
| 16. | Sandborn WJ, Hanauer S, Loftus EV, Tremaine WJ, Kane S, Cohen R, Hanson K, Johnson T, Schmitt D, Jeche R. An open-label study of the human anti-TNF monoclonal antibody adalimumab in subjects with prior loss of response or intolerance to infliximab for Crohn's disease. Am J Gastroenterol. 2004;99:1984-1989. |
| 17. | Youdim A, Vasiliauskas EA, Targan SR, Papadakis KA, Ippoliti A, Dubinsky MC, Lechago J, Paavola J, Loane J, Lee SK. A pilot study of adalimumab in infliximab-allergic patients. Inflamm Bowel Dis. 2004;10:333-338. |
| 18. | Peyrin-Biroulet L, Laclotte C, Bigard MA. Adalimumab maintenance therapy for Crohn's disease with intolerance or lost response to infliximab: an open-label study. Aliment Pharmacol Ther. 2007;25:675-680. |
| 19. | Papadakis KA, Shaye OA, Vasiliauskas EA, Ippoliti A, Dubinsky MC, Birt J, Paavola J, Lee SK, Price J, Targan SR. Safety and efficacy of adalimumab (D2E7) in Crohn's disease patients with an attenuated response to infliximab. Am J Gastroenterol. 2005;100:75-79. |