Published online Apr 28, 2007. doi: 10.3748/wjg.v13.i16.2305
Revised: December 5, 2006
Accepted: December 11, 2006
Published online: April 28, 2007
AIM: To investigate the expression of SNC73, a trans-cript of the immunoglobulin α-1 gene (IgA1-H chain), in human epithelia-derived tumor cells.
METHODS: Total RNAs and cell lysates were prepared from five different human epithelial cell lines derived from lung, stomach, liver, skin, and breast, respectively. RT-PCR and immunoblot analysis of these five cell lines were done. Both RT-PCR and immunochemistry were used to detect the expression of SNC73 in these cell lines. We also examined the expression of SNC73 in normal epithelial cells of colon mucosa by in situ hybridization. RT-PCR and immunoblot analysis were used to determine whether the recombination activating gene1/2 (RAG1 and RAG2) is present. The expression of three immunoglobulin transcription factors, EBF, E2A and Pax5, and the heavy chain of IgA1 and two types of light chains of immunoglobulin (κ and λ) in the aforementioned cell lines were analyzed by RT-PCR and immunochemistry, respectively. All the RT-PCR products were analyzed by sequencing.
RESULTS: The results of RT-PCR and immunochemistry showed that both mRNA and protein of SNC73 were expressed in five human epithelia-derived cancer cell lines. These data were further confirmed in the normal epithelial cells of colon mucosa by in situ hybridization. Also, the heavy chain of IgA1 and κ light chain were detected in these cells, but no λ light chain was obse-rved. Both RAG1 and RAG2 were expressed in these human epithelia-derived cancer cell lines and the sequence was identical to that expressed in pre-B and pre-T cells. In addition to RAG1 and RAG2, the mRNA in one of the immunoglobulin transcription factors, EBF, was also detected in these cell lines, and Pax5 was only expressed in SW480 cells, but no expression of E2A was observed in all the five cell lines.
CONCLUSION: Immunoglobulin A1 is originally expressed and V(D)J recombination machine is also present in non-lymphoid cells, suggesting that V(D)J recombination machine mediates the assembly of immunoglobulin A1 in non-lymphoid cells as in pre-lymphocytes.
- Citation: Geng LY, Shi ZZ, Dong Q, Cai XH, Zhang YM, Cao W, Peng JP, Fang YM, Zheng L, Zheng S. Expression of SNC73, a transcript of the immunoglobulin α-1 gene, in human epithelial carcinomas. World J Gastroenterol 2007; 13(16): 2305-2311
- URL: https://www.wjgnet.com/1007-9327/full/v13/i16/2305.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i16.2305
To search new molecular biomarkers related to colorectal cancer (CRC), 46 cDNA clones expressed in lower levels in CRC tissues were obtained by subtractive hybridization in Cancer Institute, Zhejiang University. By homology alignment with Genebank data using BLAST, the sequences of 32 cDNA clones share high homologies with immunoglobulins[1]. SNC73 is one of the 32 cDNA clones. Sequence analysis revealed that the full-length SNC73 cDNA is 1651 bp with an open reading frame of 1152 bp encoding an immunoglobulin α-1 molecule of 494 amino acid residues (Genebank: AF067420). The 142 amino acid residues at the N-terminal region possess high similarities with the variable regions of immunoglobulins, which contain a 20-amino acid residue signal peptide, and the 352 amino acids at the C-terminus are completely identical to the constant region of an IgA1 heavy chain (Genebank: AAC82528.1)[2]. The SNC73 gene can be mapped to human chromosome 14q32 by fluorescence in-situ hybridization. Interestingly, immunoglobulin heavy-chain genes are also located on this chromosome locus. All these results suggest that SNC73 is actually the heavy chain of IgA1 (IgHα-1).
The traditional immunological theory believes that immunoglobulins are synthesized and secreted only by B lymphocytes. Also, IgA, originally expressed in lymphocytes, is transported into epithelial cells, passages through the epithelium and enters the intestinal lumen. Interestingly, recent studies[3-5], however, also suggested that immunoglobulins are originally expressed in non-lymphoid cells. Kimoto[3] demonstrated that transcripts of Ig genes are present in four non-lymphoid tumor cell lines. Recently, Qiu et al[4] reported that epithelial cancer secretes IgG, which supports that tumor-derived IgG functions as a growth factor of epithelial cancer including carcinoma of the breast, colon, liver, lung cell lines as well as some normal tissues. Hu et al[5] found that the Tx gene, isolated from mRNA of the nasopharyngeal carcinoma cell line, CNE2, shares a high similarity with the constant region of the Ig light chain, the Kappa chain, suggesting that the Tx gene might be the Kappa chain.
All these results suggest that the heavy-chain or light-chain proteins of immunoglobulins might be expressed in non-lymphoid cells. However, there is no evidence that the entire IgA1 is expressed in epithelial cells or in cancer cells. Neither the elements of the V(D)J recombination of epithelial cells nor the recombination mechanisms have been explored so far. Here, we report that SNC73 is originally expressed in human epithelia-derived cancer cells and normal colon mucosa. We therefore examined the expression of the heavy chain of IgA-1 and two types of light chains, showing that both heavy chain of IgA1 and κ light chain are present in epithelia-derived tumor cells, but no λ light chain was observed. We also examined the expression of components of V(D)J recombination machine, showing that RAG1, RAG2 and EBF are expressed in all five epithelia-derived tumor cell lines, Pax5 was only expressed in SW480 cells, but no expression of E2A was observed. These findings show that immunoglobulin A1 is originally expressed and the V(D)J recombination machine is also present in non-lymphocytes, thus the V(D)J recombination machine mediates the maturation of immunoglobulin A1 in non-lymphocytes as in pre-lymphocytes.
Cell lines including LOVO, SW480 (both colorectal carcinoma), Hela (cervical cancer), Bcap-37 (breast cancer), SMMC-7721 (human hepatoma) were cultured in RPMI 1640 supplemented with 10% fetal bovine serum and 100 μ/mL of penicillin/streptomycin at 37°C in a humidified atmosphere containing 95% O2 and 5% CO2.
SNC73 polyclonal antibody was prepared in our laboratory. Antibodies against other proteins were obtained from commercial sources. Antibodies against RAG1, RAG2, and IgA1 were obtained from Santa Cruz. Antibodies against cytokeratin and IgHα1 were purchased from Fuzhou Maixin.
Total RNA was extracted from different cell lines with Trizol according to the manufacturer’s instructions (Life Technologies, Inc) and treated with DNaseI.
For RT-PCR analysis, 2 μg of total RNAs was reverse transcribed by M-MLV-RT (Promega) using oligo (dT)15 primer or gene-specific down-stream primers. The primers used for reverse transcriptions and PCR are shown in Table 1. PCR amplifications were performed for 40 cycles under the following conditions: denaturing at 94°C for 10 s, annealing at 58°C for 20 s, and extension at 72°C for 80 s.
| Primers for PCR of SNC73: |
| Upstream primer: 5’-CTTCCCGCTGAGCCTCTGCA-3’ |
| Downstream primer: 5’-AGCGGTCGATGGTCTTCTG-3’ |
| Primer for RT of RAG1: |
| 5’-CTGAGAATGCAGACCCGGCA-3’ |
| Primers for PCR of RAG1: |
| Upstream primer: 5’-GACATCTCAACACTTTGGCCAG-3’ |
| Downstream primer: 5’-CGGCCAGGAAGTAGCTCTC-3’ |
| Primers for PCR of RAG2: |
| Upstream primer: 5’-TACCTGGTTTAGCGGCAAAG-3’ |
| Downstream primer: 5’-CTGGCTTCAGGAACATCTCC-3’ |
| Primers for PCR of EBF: |
| Upstream primer: 5’-ACCCACGTGACATGCGGAG-3’ |
| Downstream primer: 5’-CACATAGGAGGAACAATCATGCC-3’ |
| Primer for RT of E2A: |
| 5’-CATACCTTTCACATGTGCC-3’ |
| Primers for PCR of E2A: |
| Upstream primer: 5’-GAATGAACCAGCCGCAGAGG-3’ |
| Downstream primer: 5’-AGGCCACTGTGACGTTCCTG-3’ |
| Primers for PCR of Pax5: |
| Upstream primer: 5’-GACCAGCAGGACAGGACATG-3’ |
| Downstream primer: 5’-TCCAAGGGTCAGTGACGGTC-3’ |
Cancer tissue and adjacent normal tissue were fixed in 4% polyformaldehyde within 30 min after removal from patients. Fixed samples were then embedded in paraffin, from which sections were prepared with special care to avoid RNA degradation. RNA probe was prepared by in vitro transcription of SNC73 cDNA and labeled with digoxingenin. In brief, after treatment with proteinase K and DNase I at 37°C for 15 min, sections were pre-hybridized at 42°C for 90 min in a pre-hybridization solution (4 × SSC, 50% formamide, and 0.5 mg/mL ssDNA), followed by hybridization in hybridization solution (4 × SSC, 50% formamide, 10 mg/mL ssDNA, 10% Dextron, and digoxin-labeled RNA probe) at 42°C for 4-6 h. After hybridization, sections were washed sequentially 5 times with PBS, twice with 2 × SSC, twice with 1 × SSC at 68°C, and once with buffer I (100 μmol/L Tris-HCl, pH 7.5, 150 mmol/L NaCl) at 22°C. Sections were incubated with alkaline phosphatase-conjugated anti-digoxin antibody for another 2 h at 22°C. The reactions were finally developed by addition of nitro blue tetrazolium (NBT) and 5-bromo-4 chloro-3-indolyl phosphate (BCIP).
Sections were incubated with fresh 3% hydrogen peroxide in methanol for 15 min to abolish the endogenous peroxidase activities, and then washed with PBS. Normal goat serum was used to eliminate the background before the sections were incubated with primary antibody for 2 h at room temperature. After appropriate washing, the sections were incubated with the secondary antibody at room temperature for 15 min. The sections were then treated with a streptavidin-HRP complex for 15 min and developed with 3, 3’-diaminobenzidine (DBA) and H2O2. Finally, the sections were lightly counterstained with hematoxylin. Negative control was performed by substituting the primary antibodies with pre-immune rabbit serum.
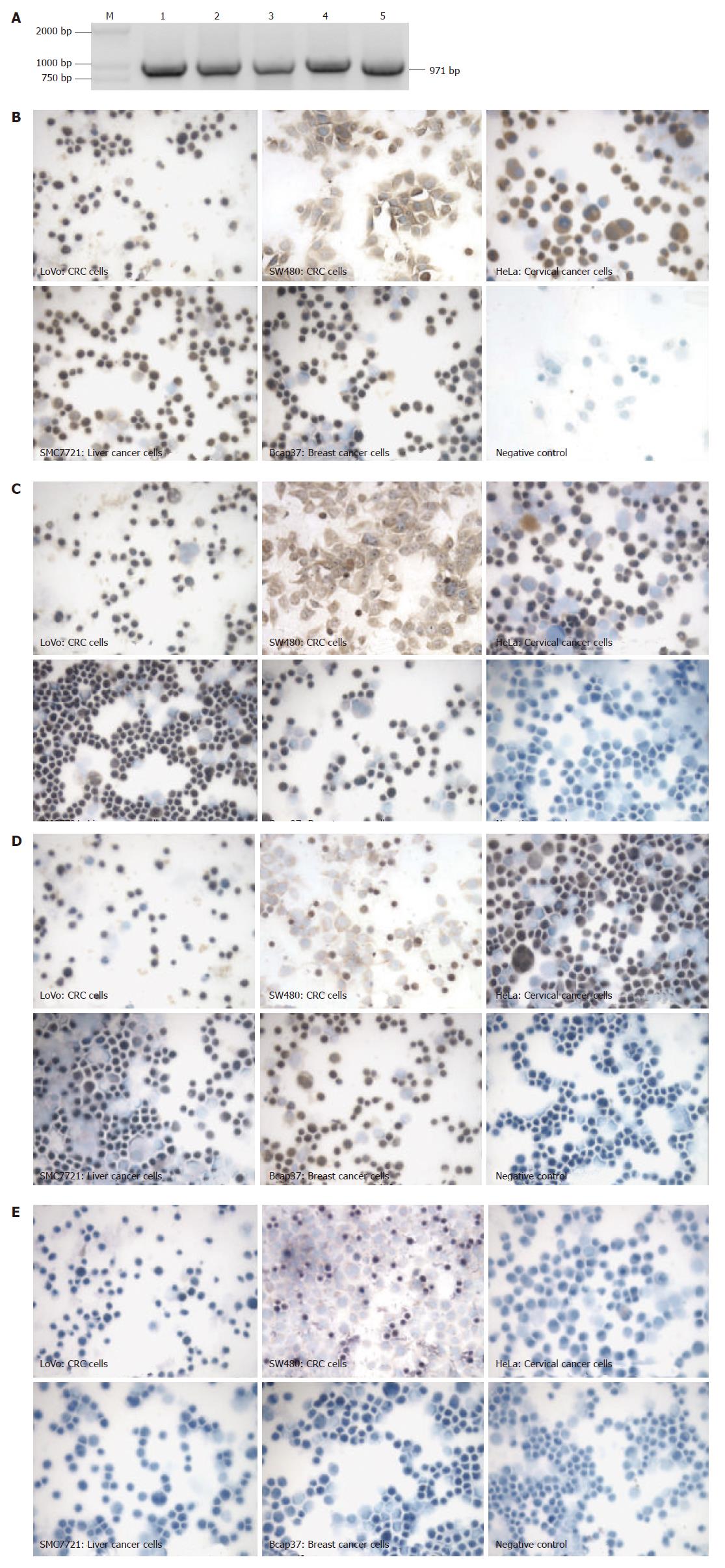
To identify whether SNC73 was originally expressed in non-lymphoid cells, we used RT-PCR and immunohisto-chemistry to examine the mRNA and protein of SNC73 in five cultured human epithelia-derived cancer cell lines (including two colorectal cancer cell lines, LoVo and SW480, one liver cancer cell line, SMC7721, one breast cancer cell line, Bcap37, and one cervical cancer cell line, HeLa). In order to eliminate the possible contamination of genomic DNA, the primers specific for SNC73 were designed in two exons separated by a 214 nt intron, the RT-PCR products from SNC73 should be 971 nucleotides. The results showed that both mRNA and protein of SNC73 were expressed in all the five cancer cell lines (Figure1A and B), and the sequencing results demonstrated that the sequence of mRNA was identical to that of SNC73 (Genebank: AF067420) (data not shown).
Since we have previously shown that SNC73 is the heavy chain of IgA1, we examined the heavy chain protein of IgA1 in these five cell lines by immunohistochemistry. Meanwhile, the proteins of immunoglobulin kappa and Lambda light-chains were also analyzed by immunohisto-chemistry. Both the heavy chain protein of IgA1 and the κ light-chain of immunoglobulin were expressed in all the five cancer cell lines (Figure 1C and D). In contrast, the Lambda light-chain of immunoglobulin was not detected (Figure1 E).
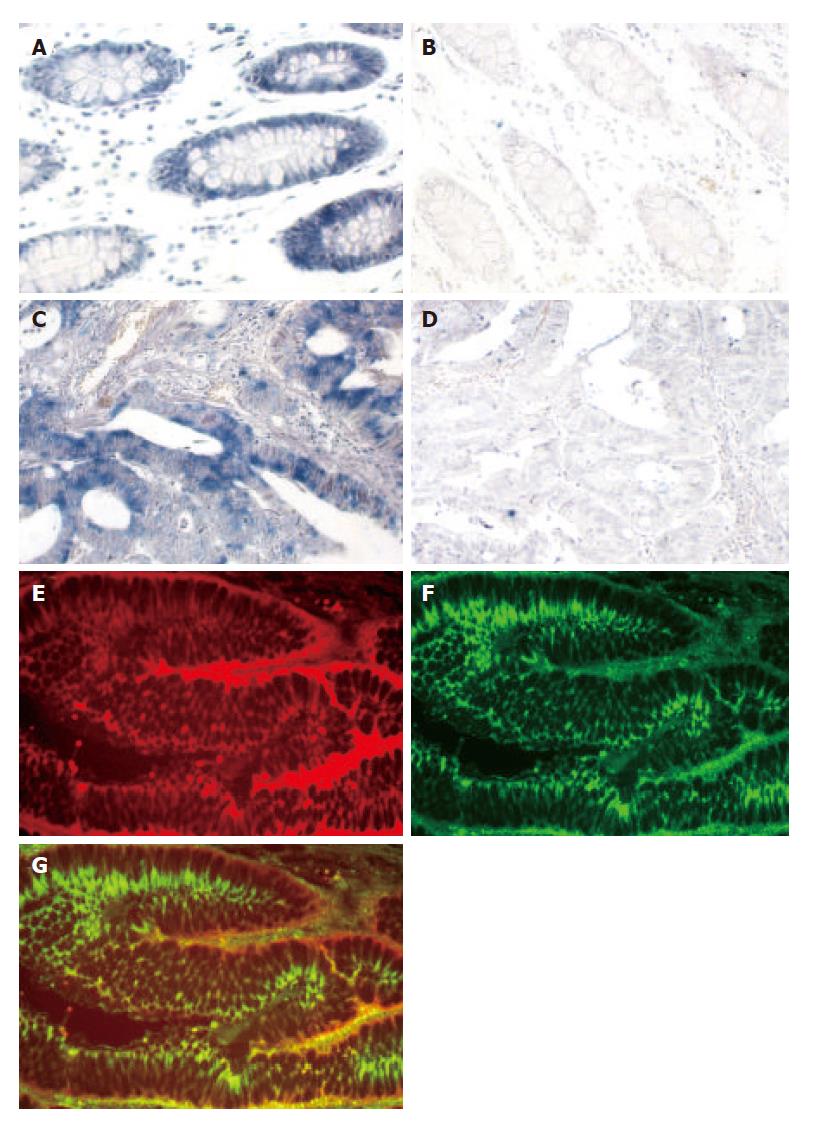
To further confirm that SNC73 is expressed in human epithelial cells, we examined the mRNA expression of SNC73 in normal colon mucosa and colon carcinoma by in situ hybridization. Blue positive signals representing the SNC73 mRNA were predominately detected in epithelial cells of normal colorectal mucosa. Faint, if any, positive signal was detected in normal lamina propria, muscular mucosa, or adventitial tissues (Figure 2A). Positive signals, which were also distributed in colorectal carcinoma (Figure 2C), appeared to be significantly weaker in most neoplastic cells as compared to those detected in normal epithelial cells. As negative controls in both normal and cancerous tissues, no positive signal was detected by hybridizing with SNC73 sense RNA probe (Figure 2B and D). To further confirm that the observed SNC73 expressed in colon mucosa is indeed the epithelial cells, we examined the cytokeratin and IgHα1 in human normal colon mucosa by double labeled-fluorescent staining immunohistochemistry. Cytokeratin is the specific marker for epithelial cells. IgHα1 and cytokeratin were co-expressed in epithelial cells (Figure 2E-G). These results suggest that SNC73 mRNA was expressed in both human normal epithelial cells and cancer cells, and its abundance was reduced in cells of colorectal carcinoma.
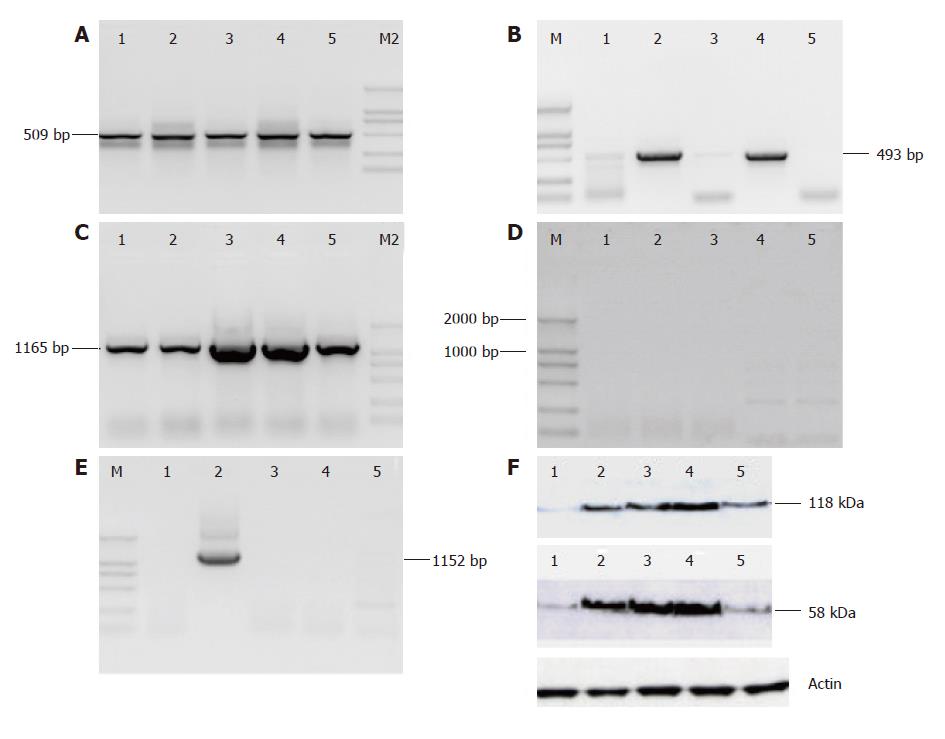
V(D)J recombination is a process by which immuno-globulin and TCR are assembled in pre-B cells and pre-T cells. Since RAG1 and RAG2 proteins form a complex to nick the DNA 5’ of the heptamer and thus generate the coding end, they play a critical role in V(D)J recombination. To substantiate the results of SNC73 expressed in human epithelial cells, we examined the transcripts and proteins of recombination activating genes, RAG-1 and RAG-2, by RT-PCR and immunoblot in five human epithelia-derived cancer cell lines expressing IgHα1 (SNC73). Interestingly, both mRNA and protein of RAG-1 and RAG-2 were observed in all the five cell lines although the expression levels were different in these cell lines (Figure 3A, B and F). The sizes of RT-PCR products from RAG1 and RAG2 were 509 bp and 493 bp, respectively. The sequences of mRNA and the molecular weight of proteins (RAG-1: 118 kDa, RAG-2: 58 kDa) were identical to those of RAG expressed in pre-B and pre-T cells.
In addition to RAG1 and RAG2, we also examined three transcription factors of immunoglobulins, EBF (early B cell factor), E2A and Pax5 by RT-PCR in the aforementioned cell lines and found that EBF transcript was present in all five cell lines, the RT-PCR products were 1165 bp, Pax5 transcript was expressed only in SW480, the band of 1152 bp. However, no E2A transcript was detected at all (Figure 3C-E). Sequencing results showed that the sequences of EBF and Pax5 expressed in epithelia-derived tumor cells were identical to those of EBF and Pax5 expressed in pre-B and pre-T cells (data not shown).
These results suggest that the entire IgA1 could be expressed in human epithelial cells, and V(D)J recombination might also be involved in its production like that in lymphocytes.
We have shown that both mRNA and protein of SNC73 are expressed in human epithelia-derived tumor cells. These results were confirmed by in situ hybridization and double-fluorescent staining of human colon mucosa. The proteins of immunoglobulin A1 heavy chain (IgHα1), Igκ, and Igλ were analyzed in five cancer cell lines by immunohistochemistry, showing that IgHα1 and Igκ were expressed in all five cancer cell lines, except for Igλ. These findings suggest that the entire IgA1 is expressed in human epithelial cells and support our previous hypothesis that immunoglobulin A1 might be expressed in non-lymphoid cells[2].
V(D)J recombination is a process by which immu-noglobulin and TCR are assembled in pre-B and pre-T cells, and is only present in lymphocytes. Its two major components, RAG1 and RAG2, are only expressed in pre-B and pre-T cells. Our findings reported here, however, suggest that the V(D)J recombination machine is present in epithelia-derived tumor cells, similar to previous findings in the murine central nervous system[6]. In addition to RAG1 and RAG2, the immunoglobulin transcription factor EBF was expressed in all five epithelial-derived cell lines and Pax5 was only expressed in SW480 cells in this study, suggesting that the mechanism of V(D)J recombination in these epithelia-derived tumor cell lines is slightly different. It was reported that co-expression of EBF/RAG and E2A/RAG in non-lymphoid cells induces Lambda light chain recombination and Kappa light chain recombination, respectively[7]. However, in the present study, all the five cell lines expressed endogenous EBF and RAG expressed the Kappa light chain but not the Lambda light chain. This discrepancy may be due to the functional difference between the endogenous EBF/RAG and co-expressed EBF/RAG.
S- Editor Liu Y L- Editor Wang XL E- Editor Ma WH
| 1. | Cao J, Cai X, Zheng L, Geng L, Shi Z, Pao CC, Zheng S. Characterization of colorectal-cancer-related cDNA clones obtained by subtractive hybridization screening. J Cancer Res Clin Oncol. 1997;123:447-451. |
| 2. | Zheng S, Cao J, Geng L. [Structure and expression of colorectal cancer related Immunoglobulin novel gene SNC73]. Zhonghua YiXue ZaZhi. 2001;81:485-488. |
| 3. | Kimoto Y. Expression of heavy-chain constant region of immunoglobulin and T-cell receptor gene transcripts in human non-hematopoietic tumor cell lines. Genes Chromosomes Cancer. 1998;22:83-86. |
| 4. | Qiu X, Zhu X, Zhang L, Mao Y, Zhang J, Hao P, Li G, Lv P, Li Z, Sun X. Human epithelial cancers secrete immunoglobulin g with unidentified specificity to promote growth and survival of tumor cells. Cancer Res. 2003;63:6488-6495. |
| 5. | Hu WX, Cao Y, Li XY, Lee LM, Yao KT. Comparison of NPC Transforming gene Tx to Ig Kappa constant region gene and their expression in different cell lines. Acta Biochim Biophy Sin. 1995;27:215-221. |