Published online Apr 28, 2007. doi: 10.3748/wjg.v13.i16.2298
Revised: December 29, 2006
Accepted: February 15, 2007
Published online: April 28, 2007
AIM: To investigate the effect of selective Cycloo-xygenase-2 (COX-2) inhibitor 4-[5-(4-Chloro-phenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl] benzenesulfonamide (SC-236), on the cholecystokinin (CCK)-octapeptide-induced acute pancreatitis (AP) in rats.
METHODS: Wistar rat weighing 240 g to 260 g were divided into three groups. (1) Normal DMSO treated group, (2) SC-236 at 4 mg/kg treated group; SC-236 systemically administered via the intravenous (i.v.) catheter, followed by 75 μg/kg CCK octapeptide subcutaneously three times, after 1, 3 and 5 h. This whole procedure was repeated for 5 d. (3) Dimethyl sulfoxide (DMSO) treated group: an identical protocol was used in this group as in the SC-236 cohort (see 2. above). Repeated CCK octapeptide treatment resulted in a typical experimentally induced pancreatitis in the Wistar rats.
RESULTS: SC-236 improved the severity of CCK-octapeptide-induced AP as measured by laboratory criteria [the pancreatic weight/body weight (p.w/b.w) ratio, the level of serum amylase and lipase]. The SC-236 treated group showed minimal histologic evidence of pancreatitis and a significant reduction in myeloperoxidase activity. SC-236 also increased heat shock protein (HSP)-60 and HSP72 compared with the DMSO-treated group in the CCK-octapeptide-induced AP and also reduced the pancreatic levels of COX-2. Furthermore, SC-236 reduced proinflammatory cytokine synthesis and inhibited NF-κB activation compared with the DMSO-treated group in the CCK-octapeptide-induced AP.
CONCLUSION: Our results suggested that COX-2 plays pivotal role in the development of AP and COX-2 inhibitors may play a beneficial role in preventing AP.
- Citation: Seo SW, Jung WS, Piao TG, Hong SH, Yun KJ, Park RK, Shin MK, Song HJ, Park SJ. Selective cyclooxygenase-2 inhibitor ameliorates cholecystokinin-octapeptide-induced acute pancreatitis in rats. World J Gastroenterol 2007; 13(16): 2298-2304
- URL: https://www.wjgnet.com/1007-9327/full/v13/i16/2298.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i16.2298
Acute pancreatitis (AP) is characterized by local pancreatic inflammation as well as a systemic inflammatory response[1,2]. Histologically, AP is characterized by interstitial edema, vacuolization, inflammation, and acinar cell necrosis[3,4]. A widely used model to study these responses is AP induced in rats and mice by high doses of cholecystokinin (CCK) or its analogue, cerulein[5]. CCK is a major physiologic regulator of digestive enzyme secretion by the pancreatic acinar cell; however, supraphysiological doses of CCK (in excess of those that stimulate maximal secretion of digestive enzymes) can cause injury to the pancreas, causing pancreatitis that mimics many features of the human disease[5-8]. However, the factors that determine the development and severity of pancreatitis are not fully understood.
Cyclooxygenase (COX), a key enzyme in the synthesis of prostaglandins (PGs) from arachidonic acid, exists in two isoforms: COX-1 and COX-2. Cyclooxygenase-1 is constitutively expressed in all tissues and regulates the actions of PGs, whereas COX-2 is induced in immune cells and other tissues following diverse stimuli, such as mitogens, proinflammatory cytokines, growth factors, and tumor promoters[9]. COX-2 also regulates PGs, which are important in many reproductive processes[10]. Recently, it was reported that COX-2 is a crucial and central mediator in the development and severity of acute pancreatitis[11]. Expression of COX-2, the inducible form of the COX enzyme, is normally undetectable in pancreatic acinar cells[11]. However, COX-2 is markedly induced in AP[11,12]. COX-2 expression is regulated, in part, by NF-κB, certain mitogens, and the proinflammatory cytokines interleukin (IL)-1, IL-6, and tumor necrosis factor (TNF)[9,13]. COX-2 inhibitors, a group of specific nonsteroidal anti-inflammatory drugs (NSAIDS), are widely used as anti-inflammatory agents and as analgesia for various disorders. Selective blockage of COX-2 using pharmacologic agents reduces PG production and ovulation both in vivo and in vitro in the rat[14]. Selective COX-2 inhibitors are used for the treatment of inflammation and pain with the advantage of having fewer upper gastrointestinal adverse effects compared to traditional nonsteroidal anti-inflammatory drugs[15].
The aim of the present study was to investigate the potential effects of SC-236 on the severity of CCK octapeptide-induced AP. Moreover, we wished to evaluate whether SC-236 can inhibit the pancreatic NF-κB DNA binding activity and proinflammatory cytokine synthesis during CCK octapeptide-induced AP. Additionally, we set out to investigate the potential effects of SC-236 and CCK octapeptide on pancreatic HSP60 and HSP72 synthesis.
Avidin-peroxidase and 2'-AZINO-bis (3-ethylbenzithia-zoline-6-sulfonic acid) tablets substrate and CCK-8 were purchased from Sigma (St. Louis, MO, USA). Anti-HSP60, anti-HSP72 and anti-COX-2 antibodies were purchased from Stressgen (British Columbia, Canada). Anti-rat TNF-α, anti-rat IL-1β and anti-rat IL-6 antibodies were purchased from R&D Systems (Minneapolis, MN, USA). SC-236 and SC-58125 were obtained from Calbiochem (La Jolla, CA).
Male Wistar rats weighing 240-260 g were used. The animals were kept at a constant room temperature of 25°C with a 12 h light-dark cycle, and allowed free access to water and standard laboratory foodstuff. The rats were fasted for 16 h before the induction of AP. In each experimental group 6-10 rats were used. The experiments performed in this study were approved by the Animal Care Committee of the Wonkwang University.
CCK octapeptide (75 μg/kg) or vehicle control was administered to Male Wistar rats weighing 240-260 g subcutaneously three times, at 1, 3, and 5 h as previously described[16]. This whole procedure was repeated for 5 d. The animals were sacrificed by exsanguinations through the abdominal aorta 12 h after the last CCK-octapeptide injection and the blood and pancreas were harvested for measurements. The pancreas was quickly removed, cleaned from fat and lymph node, weighed, and frozen at -70°C until use. Rats were treated in accordance with the current law and the NIH Guide for Care and Use of Laboratory Animals.
SC-236-treated group, 4 mg/kg SC-236 was systemically administrated via the intravenous (i.v.) catheter, followed by 75 μg/kg CCK-octapeptide subcutaneously three times, after 1, 3, and 5 h. This whole procedure was repeated for 5 d. Due to the extremely poor solubility in aqueous solvents, the SC-236 was dissolved in dimethyl sulfoxide (DMSO) for systemic administration. The animals were sacrificed by exsanguinations through the abdominal aorta 12 h after the last CCK-octapeptide injection and the blood and pancreas were harvested for measurements.
This ratio was utilized to evaluate the degree of pancreatic edema.
Western blot analysis of pancreatic HSP60, HSP72 and COX-2 was performed from the cytosolic fraction of the pancreas homogenates. Thirty micrograms of protein were loaded per lane. Samples were electrophoresed on a 10% SDS-PAGE according to the method of Laemmli[17]. The gels were either stained with Coomassie brilliant blue (to demonstrate equal loading of proteins for Western blot analysis) or transferred to a nitrocellulose membrane for 2 h at 300 mA. Membranes were blocked in 5% non-fat dry milk for 1 h and incubated with anti-HSP60, anti-HSP72 and anti-COX-2 antibodies. After washing in PBS-Tween-20 three times, the blot was incubated with secondary antibody for 30 min and the antibody-specific proteins were visualized by the enhanced chemiluminesence detection system according to the recommended procedure (Amersham Corp. Newark, NJ).
ELISA for TNF-α, IL-1 and IL-6 (R&D Systems, Minneapolis, MN, USA) was carried out in duplicate in 96-well plates (Nunc, Denmark) coated with each of 100 μL aliquots of anti-rat IL-6, IL-1 and TNF-α monoclonal antibodies at 1.0 μg/mL in PBS at pH 7.4 and was incubated overnight at 4°C. The plates were washed in PBS containing 0.05% Tween-20 (Sigma, St. Louis, MO, USA) and blocked with PBS containing 1% BSA, 5% sucrose and 0.05% NaN3 for 1 h. After additional washes, standards were added and incubated at 37°C for 2 h. After 2 h incubation at 37°C, the wells were washed and then each of 0.2 μg/mL of biotinylated anti-rat TNF-α, IL-1 and IL-6 were added and again incubated at 37°C for 2 h. After the wells were washed, avidin-peroxidase was added and plates were incubated for 20 min at 37°C. Wells were again washed and ABTS substrate was added. Color development was measured at 405 nm using an automated microplate ELISA reader. A standard curve was run on each assay plate using recombinant TNF-α, IL-1 and IL-6 in serial dilutions.
Arterial blood samples for determinations of serum amylase and lipase were obtained 12 h after pancreatitis induction. Rats were anesthetized with intraperitoneal injection of ketamine (80 mg/kg) and xylazine (4 mg/kg). After anesthetization, bloods were withdrawn from the heart into syringes. Serum amylase was measured by using ADIVA 1650 (BAYER, USA). Serum lipase was measured by using a Cobas-mira (Roche, USA).
Nuclear extracts were prepared as described by Dyer and Herzog[18]. Reaction mixtures (25 μL, pH 7.5) contained 7.5-10 μg of nuclear protein, 5 mmol/L Tris, 100 mmol/L NaCl, 1 mmol/L dithiothreitol, 1 mmol/L EDTA, 4% (vol/vol) glycerol, and 0.08 mg/mL salmon sperm DNA. The oligonucleotide probe [5'-AGT TGA GGG GAC TTT CCC AGG C-3' (Promega, Madison, WI)] containing the κB binding motif was end-labeled with [γ-32P]ATP using T4 polynucleotide kinase and purified over two successive 1-mL G-50 columns (Amersham Pharmacia Biotech). The probe (1 × 106 counts/min) was added to the reaction mixture, and the binding reaction was allowed to proceed for 20 min at room temperature. DNA-protein complexes were resolved in a 6% nondenaturing polyacrylamide gel in buffer containing 22.5 mmol/L Tris, 22.5 mmol/L boric acid, and 0.5 mmol/L EDTA, pH 8.3, at 140 V for 2-3 h. Gels were dried and exposed to Kodak Bio Max MR films at -70°C. NF-κB bands from films were quantitated by using a Hewlett-Packard Scanjet 4100 scanner and a Scion image-analysis program.
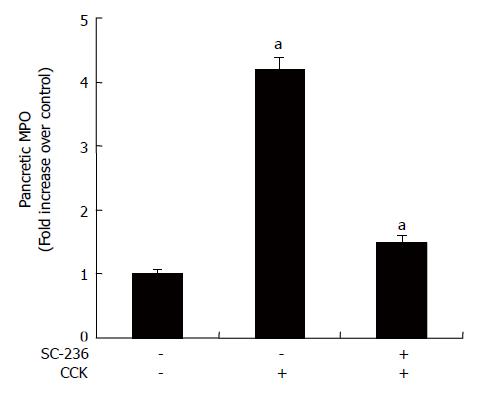
Neutrophil sequestration in pancreas was quantified by measuring tissue myeloperoxidase (MPO) activity[11,12]. Tissue samples were thawed, homogenized in 20 mmol/L phosphate buffer (pH 7.4), and centrifuged (10 000 ×g, 10 min, 4°C), and the resulting pellet was resuspended in 50 mmol/L phosphate buffer (pH 6.0) containing 0.5% hexadecyltrimethylammonium bromide (Sigma). The suspension was subjected to four cycles of freezing and thawing and was further disrupted by sonication (40 s). The sample was then centrifuged (10 000 ×g, 5 min, 4°C), and the supernatant used for the MPO assay. The reaction mixture consisted of the supernatant, 1.6 mmol/L tetramethylbenzidine (Sigma), 80 mmol/L sodium phosphate buffer (pH 5.4), and 0.3 mmol/L hydrogen peroxide. This mixture was incubated at 37°C for 110 s, the reaction was terminated with 2 mol/L H2SO4, and the absorbance was measured at 450 nm. This absorbance was then corrected for the DNA content of the tissue sample (fold increase over control).
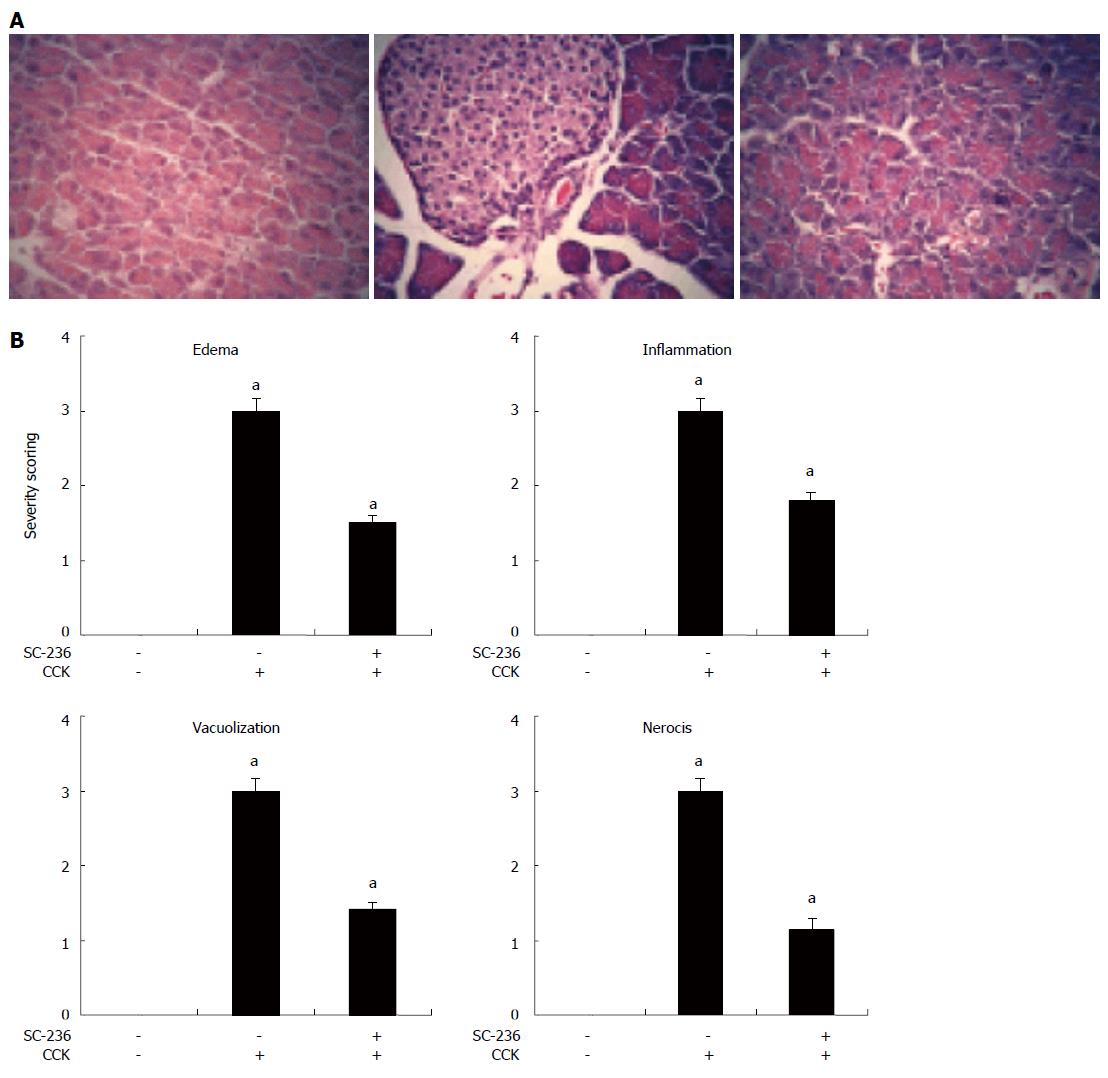
The entire pancreas of at least 5 rats from each treatment group was examined and semiquantitated based on necrosis, vacuolization, inflammation, and edema by a pathologist who was blinded to the treatment. Using a previously described method by Schmidt et al. The entire sections (a minimum of 100 fields) were examined for each sample and scored on a scale of 0-3 (0 being normal and 3 being severe) based on the number of acinar cell necrosis and the presence of vacuolization, interstitial edema, and interstitial inflammation and to what extent these characteristics affected the organ. These characteristics include the presence of acinar-cell ghosts, vacuolization and swelling of the acinar cells, and/or the destruction of the histoarchitecture of whole or parts of the acini.
Results were expressed as means ± SE. The significance of changes was evaluated using student's t test. Differences between the experimental groups were evaluated by using analysis of variance. Values of P < 0.05 were accepted as statistically significant.
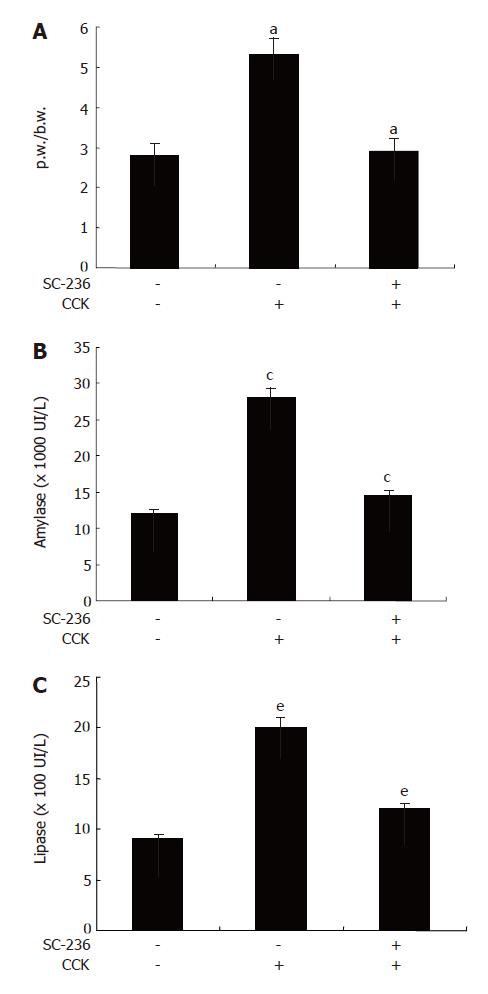
It has been shown that p.w/b.w is increased in CCK-octapeptide-induced pancreatitis in rats[16]. To assess the effect of SC-236 on p.w/b.w during CCK octapeptide-induced AP pancreatic weight was divided by the body weight of the rat. As shown in Figure 1A, SC-236 significantly decreased p.w/b.w compared with DMSO-treated group (2.912 ± 0.13 vs 5.3 ± 0.19) (P < 0.05). Levels of serum amylase and lipase are most commonly obtained as biochemical markers of pancreatic disease, particularly in AP. Amylase activity in serum has been used for many years for the evaluation of patients with acute abdominal pain and suspected pancreatic disorders[19,20]. Elevation of pancreatic lipase levels in plasma or serum is often considered to be the most sensitive and specific marker of AP[21]. As shown in Figure 1B, C, SC-236 significantly decreased the levels of serum amylase and lipase in the CCK-octapeptide-induced AP compared with DMSO.
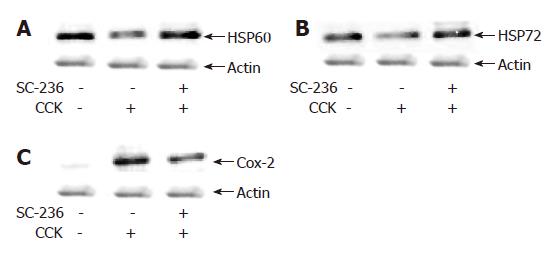
It was reported that the preinduction of HSP expression has a protective effect against cerulein-induced pancreatitis in rats or choline-deficient ethionine-supplemented diet model pancreatitis in mice[16,22]. Next we investigated the expression of pancreatic HSP60 and HSP72 in CCK octapeptide-induced AP. As shown in Figure 2A and B, SC-236 increased the expression of pancreatic HSP60 and HSP72 compared with the DMSO-treated group. Furthermore, we also examined the expression of COX-2. In the accordance with previous reports, as shown in Figure 2C, COX-2 is markedly induced with AP[11,12]. SC-236 resulted in a decrease in pancreatic COX-2 expression in CCK-octapeptide-induced AP (Figure 2C).
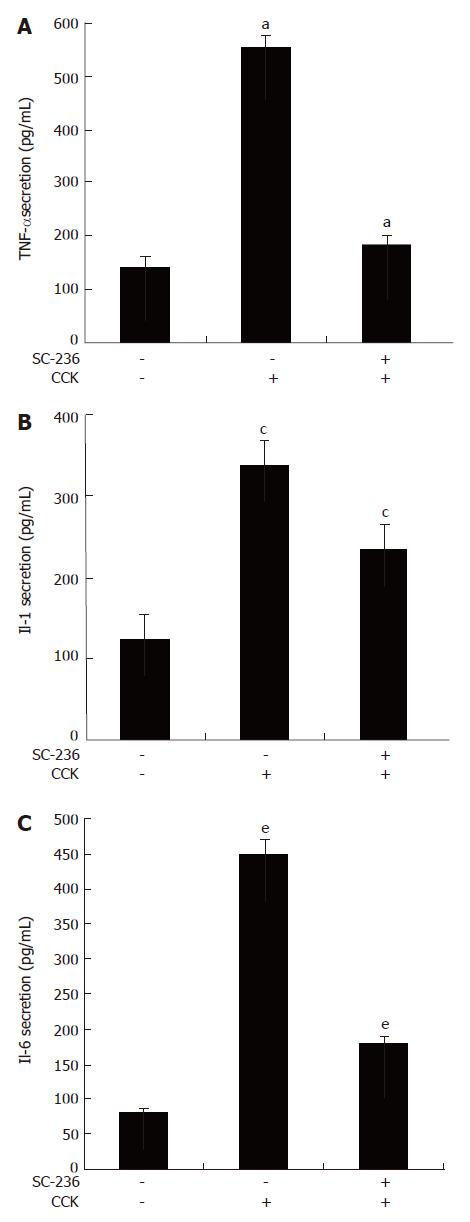
TNF-α, IL-1 and IL-6 production were increased in serum during CCK octapeptide-induced AP[23-25]. We examined whether SC-236 reduces production of several pro-inflammatory cytokines such as TNF-α, IL-1 and IL-6. SC-236 pretreatment decreased the level of TNF-α (52.7 ± 6.43 pg/mL), IL-1 (125 ± 13.7 pg/mL) and IL-6 production (180 ± 13 pg/mL) during CCK-octapeptide-induced AP (Figure 3).
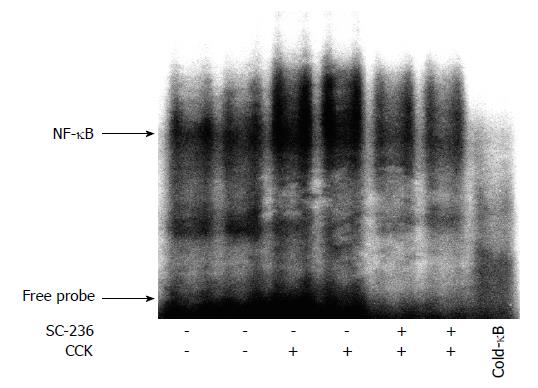
It was reported that NF-κB activation was important for the production of cytokines such as TNF-α, IL-1 and IL-6[26]. We examined whether SC-236 inhibits the activation of NF-κB during CCK octapeptide-induced AP. As shown in Figure 4, SC-236 significantly inhibits NF-κB activation.
As an additional quantitative assessment of the severity of the inflammatory response, we measured MPO activity, an indicator of neutrophil sequestration in AP. As shown in Figure 5, MPO activity was suppressed after the CCK octapeptide injection when SC-236 was applied.
The next step taken to determine whether SC-236 protects against the progression and severity of pancreatitis was histological examination of the pancreas. Histologic sections from representative pancreas are shown in Figure 6A. Control animals showed healthy non-edematous acinar cells (left panel), whereas treatment with CCK octapeptide resulted in marked necrosis, vacuolization, and inflammatory cell sequestration (middle panel). Treatment with SC-236 (right panel) attenuated the severity of the pancreatitis as noted by decreased interstitial edema and reduced polymorphonuclear granulocyte sequestration. To assess these changes in a semiquantitative fashion, histologic slides of the pancreatic tissues were scored as described in the Materials and Methods section. Treatment with SC-236 significantly reduced the severity of the pancreatitis, as noted by decreases in pancreatic edema, inflammation, vacuolization, and necrosis (Figure 6B).
Recently it was reported that COX-2 is a crucial and central mediator in the development and severity of acute pancreatitis[11,12]. In this study, we sought to determine whether selective COX-2 inhibitor SC-236 could ameliorate CCK octapeptide-induced AP. We found that SC-236 ameliorated CCK-induced AP. These results suggest that COX-2 is a crucial and central mediator in the development and severity of acute pancreatitis. Cyclooxygenases as the key enzymes of prostaglandin synthesis have an important role in the regulation of inflammation. may also have other important proinflammatory effects that do not involve prostanoid synthesis[27]. Much is known regarding the proinflammatory role of in a variety of and chronic infla-mmatory conditions including asthma, arthritis, and the inflammatory bowel diseases[10].
Selective COX-2 inhibitors and NSAIDs have also been tested for their therapeutic efficacy when combined with chemotherapeutic drugs or radiation[28]. A number of in vitro studies showed that these agents can act synergistically, additively, or supra-additively when combined with some chemotherapeutic agents[29].
Previous report indicated that selective COX-2 inhibitor significantly enhanced tumor response to radiation, determined either by tumor growth delay or tumor cure compared with other COX-2 inhibitors[30]. In the accordance with others reports, previous reports indicated that the selective COX-2 inhibitor, SC-58125, decreased inflammation and NF-κB activation in cerulein-induced AP[11,12]. In the accordance with these papers, our data have shown that SC-236 also ameliorated CCK-induced AP.
The heat shock proteins (HSPs) play a universal role in the maintenance of cellular homeostasis[26]. They are expressed constitutively and/or at elevated levels upon the exposure of cells to a variety of stress conditions in every organ, including the pancreas[31]. It has been shown that the pre-induction of HSP expression has a protective effect against cerulein-induced pancreatitis in rats or in the choline-deficient ethionine-supplemented diet model of pancreatitis in mice[22]. Whereas many diseases result in increased levels of HSPs, Strowski et al[32] demonstrated that cerulein-induced pancreatitis reduces the levels of pancreatic HSPs. This observation even suggests that the low levels of pancreatic HSPs might be involved in the development of CCK octapeptide-induced pancreatitis. Moreover, an increasing body of evidence from experi-mental animal studies has documented an essential role of HSPs in the prevention of AP. In accordance with Strowski et al[32], we have shown that supramaximal doses of CCK octapeptide reduce the levels of HSP60 and HSP72. However, this decrease was recovered by the administration of the SC-236.
TNF-α and IL-1β are thought to mediate the systemic effects of pancreatitis such as fever, hypotension, and shock[14]. Serum levels of TNF-α and IL-1β were used to evaluate the systemic cytokine response. TNF-α has been shown to be an important initiator of the local and systemic damage occurring in AP[33]. Additionally, serum levels of TNF-α have been found to correlate with severity of AP in humans[23]. IL-1 has been shown to be a significant cytokine for the development of AP. Inhibition of cytokine production has been proposed to decrease the severity of acute pancreatitis[24,25]. In contrast to the findings of Micbele et al (2004), our data demonstrated that SC-236 reduced TNF-α, IL-1 and IL-6 production on CCK octapeptide-induced AP in rats. There are several different experimental factors between the studies of Slogoff MI et al[19] and our studies including the animals, COX-2 inhibi-tors and cytokine detection time. SC-236 pretreatment reduced the examined laboratory and biochemical parameters in the CCK octapeptide-induced AP in rats.
In conclusion, this study showed that SC-236 pre-treatment ameliorated the severity of CCK octapeptide-induced AP in rats. In agreement with others, we have demonstrated that SC-236 increased the synthesis of HSP60 and HSP72, and decreased expression of COX-2 and reduced TNF-α, IL-1 and IL-6 production in CCK octapeptide-induced AP in rats. Additionally SC-236 pretreatment ameliorated many of the examined laboratory and biochemical parameters of the disease. Our findings suggest that SC-236 may be a beneficial target of acute pancreatitis.
S- Editor Liu Y L- Editor Anand AS E- Editor Ma WH
| 1. | Baron TH, Morgan DE. Acute necrotizing pancreatitis. N Engl J Med. 1999;340:1412-1417. |
| 2. | Bhatia M, Brady M, Zagorski J, Christmas SE, Campbell F, Neoptolemos JP, Slavin J. Treatment with neutralising antibody against cytokine induced neutrophil chemoattractant (CINC) protects rats against acute pancreatitis associated lung injury. Gut. 2000;47:838-844. |
| 3. | O'Konski MS, Pandol SJ. Effects of caerulein on the apical cytoskeleton of the pancreatic acinar cell. J Clin Invest. 1990;86:1649-1657. |
| 4. | Jungermann J, Lerch MM, Weidenbach H, Lutz MP, Krüger B, Adler G. Disassembly of rat pancreatic acinar cell cytoskeleton during supramaximal secretagogue stimulation. Am J Physiol. 1995;268:G328-G338. |
| 5. | Lerch MM, Adler G. Experimental animal models of acute pancreatitis. Int J Pancreatol. 1994;15:159-170. |
| 6. | Steer ML. Early events in acute pancreatitis. Baillieres Best Pract Res Clin Gastroenterol. 1999;13:213-225. |
| 7. | Lerch MM, Gorelick FS. Early trypsinogen activation in acute pancreatitis. Med Clin North Am. 2000;84:549-563, viii. |
| 8. | Norman J. The role of cytokines in the pathogenesis of acute pancreatitis. Am J Surg. 1998;175:76-83. |
| 9. | Schmedtje JF, Ji YS, Liu WL, DuBois RN, Runge MS. Hypoxia induces cyclooxygenase-2 via the NF-kappaB p65 transcription factor in human vascular endothelial cells. J Biol Chem. 1997;272:601-608. |
| 10. | Roberts PJ, Morgan K, Miller R, Hunter JO, Middleton SJ. Neuronal COX-2 expression in human myenteric plexus in active inflammatory bowel disease. Gut. 2001;48:468-472. |
| 11. | Ethridge RT, Chung DH, Slogoff M, Ehlers RA, Hellmich MR, Rajaraman S, Saito H, Uchida T, Evers BM. Cyclooxygenase-2 gene disruption attenuates the severity of acute pancreatitis and pancreatitis-associated lung injury. Gastroenterology. 2002;123:1311-1322. |
| 12. | Song AM, Bhagat L, Singh VP, Van Acker GG, Steer ML, Saluja AK. Inhibition of cyclooxygenase-2 ameliorates the severity of pancreatitis and associated lung injury. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1166-G1174. |
| 13. | Yamamoto K, Arakawa T, Ueda N, Yamamoto S. Transcriptional roles of nuclear factor kappa B and nuclear factor-interleukin-6 in the tumor necrosis factor alpha-dependent induction of cyclooxygenase-2 in MC3T3-E1 cells. J Biol Chem. 1995;270:31315-31320. |
| 14. | Norman RJ, Wu R. The potential danger of COX-2 inhibitors. Fertil Steril. 2004;81:493-494. |
| 15. | Foral PA, Nystrom KK, Wilson AF, Christensen CM. Gastrointestinal-related adverse effects of COX-2 inhibitors. Drugs Today (Barc). 2003;39:939-948. |
| 16. | Seo SW, Koo HN, An HJ, Kwon KB, Lim BC, Seo EA, Ryu DG, Moon G, Kim HY, Kim HM. Taraxacum officinale protects against cholecystokinin-induced acute pancreatitis in rats. World J Gastroenterol. 2005;11:597-599. |
| 17. | Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680-685. |
| 18. | Dyer RB, Herzog NK. Immunodepletion EMSA: a novel method to identify proteins in a protein-DNA complex. Nucleic Acids Res. 1995;23:3345-3346. |
| 19. | Slogoff MI, Ethridge RT, Rajaraman S, Evers BM. COX-2 inhibition results in alterations in nuclear factor (NF)-kappaB activation but not cytokine production in acute pancreatitis. J Gastrointest Surg. 2004;8:511-519. |
| 20. | Panozzo MP, Basso D, Fabris C, Faggian D, Meggiato T, Plebani M, Del Favero G, Fogar P, Scalon P, Ferrara C. Diagnostic utility of a new monoclonal antibody pancreatic isoamylase assay in chronic pancreatic diseases. J Clin Chem Clin Biochem. 1990;28:485-488. |
| 21. | Panteghini M, Pagani F, Bonora R. Clinical and analytical evaluation of a continuous enzymatic method for measuring pancreatic lipase activity. Clin Chem. 1993;39:304-308. |
| 22. | Wagner AC, Weber H, Jonas L, Nizze H, Strowski M, Fiedler F, Printz H, Steffen H, Göke B. Hyperthermia induces heat shock protein expression and protection against cerulein-induced pancreatitis in rats. Gastroenterology. 1996;111:1333-1342. |
| 23. | Exley AR, Leese T, Holliday MP, Swann RA, Cohen J. Endotoxaemia and serum tumour necrosis factor as prognostic markers in severe acute pancreatitis. Gut. 1992;33:1126-1128. |
| 24. | Gross V, Leser HG, Heinisch A, Schölmerich J. Inflammatory mediators and cytokines--new aspects of the pathophysiology and assessment of severity of acute pancreatitis? Hepatogastroenterology. 1993;40:522-530. |
| 25. | Heath DI, Cruickshank A, Gudgeon M, Jehanli A, Shenkin A, Imrie CW. Role of interleukin-6 in mediating the acute phase protein response and potential as an early means of severity assessment in acute pancreatitis. Gut. 1993;34:41-45. |
| 26. | Rakonczay Z, Duda E, Kaszaki J, Iványi B, Boros I, Lonovics J, Takács T. The anti-inflammatory effect of methylprednisolone occurs down-stream of nuclear factor-kappaB DNA binding in acute pancreatitis. Eur J Pharmacol. 2003;464:217-227. |
| 27. | de Gregorio R, Iñiguez MA, Fresno M, Alemany S. Cot kinase induces cyclooxygenase-2 expression in T cells through activation of the nuclear factor of activated T cells. J Biol Chem. 2001;276:27003-27009. |
| 28. | Koki AT, Leahy KM, Masferrer JL. Potential utility of COX-2 inhibitors in chemoprevention and chemotherapy. Expert Opin Investig Drugs. 1999;8:1623-1638. |
| 29. | Hida T, Kozaki K, Muramatsu H, Masuda A, Shimizu S, Mitsudomi T, Sugiura T, Ogawa M, Takahashi T. Cyclooxygenase-2 inhibitor induces apoptosis and enhances cytotoxicity of various anticancer agents in non-small cell lung cancer cell lines. Clin Cancer Res. 2000;6:2006-2011. |
| 30. | You HJ, Mørch CD, Chen J, Arendt-Nielsen L. Differential antinociceptive effects induced by a selective cyclooxygenase-2 inhibitor (SC-236) on dorsal horn neurons and spinal withdrawal reflexes in anesthetized spinal rats. Neuroscience. 2003;121:459-472. |
| 31. | Schäfer C, Williams JA. Stress kinases and heat shock proteins in the pancreas: possible roles in normal function and disease. J Gastroenterol. 2000;35:1-9. |
| 32. | Strowski MZ, Sparmann G, Weber H, Fiedler F, Printz H, Jonas L, Göke B, Wagner AC. Caerulein pancreatitis increases mRNA but reduces protein levels of rat pancreatic heat shock proteins. Am J Physiol. 1997;273:G937-G945. |
| 33. | Lane JS, Todd KE, Gloor B, Chandler CF, Kau AW, Ashley SW, Reber HA, McFadden DW. Platelet activating factor antagonism reduces the systemic inflammatory response in a murine model of acute pancreatitis. J Surg Res. 2001;99:365-370. |