Published online Apr 28, 2007. doi: 10.3748/wjg.v13.i16.2283
Revised: February 3, 2007
Accepted: February 14, 2007
Published online: April 28, 2007
AIM: To investigate the relationship between cycloo-xygenase-2 (COX-2), and vascular endothelial growth factor (VEGF), and to determine the clinical significance of this relationship in esophageal cancer patients undergoing chemoradiotherapy (CRT).
METHODS: Immunohistochemical staining was used to evaluate COX-2 and VEGF expression in 40 patients with histologically-confirmed esophageal squamous carcinoma (ESCC) who were undergoing preoperative CRT.
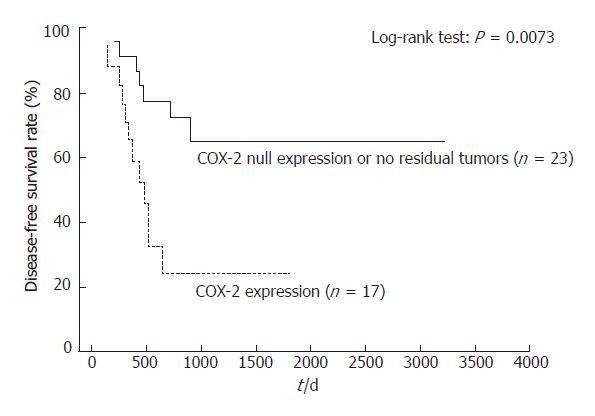
RESULTS: Fourteen out of 40 ESCC patients showed a pathological complete response (CR) after CRT. COX-2 and VEGF protein expressions were observed in the cytoplasm of 17 and 13 tumors, respectively, with null expression in 9 and 13 tumors, respectively. COX-2 expression was strongly correlated with VEGF expression (P < 0.05). There were also significant associations between COX-2 expression, tumor recurrence, and lymph-node involvement (P = 0.0277 and P = 0.0095, respectively). COX-2 expression and VEGF expression had significant prognostic value for disease-free survival (log-rank test; P = 0.0073 and P = 0.0341, respectively), but not for overall survival, as assessed by univariate analysis.
CONCLUSION: Our results suggest that COX-2 expression correlates with VEGF expression and might be a useful prognostic factor for more frequent tumor recurrence in ESCC patients undergoing neoadjuvant CRT. These findings support the use of anti-angiogenic COX-2 inhibitors in the treatment of ESCC.
- Citation: Yoshikawa R, Fujiwara Y, Koishi K, Kojima S, Matsumoto T, Yanagi H, Yamamura T, Hashimoto-Tamaoki T, Nishigami T, Tsujimura T. Cyclooxygenase-2 expression after preoperative chemoradiotherapy correlates with more frequent esophageal cancer recurrence. World J Gastroenterol 2007; 13(16): 2283-2288
- URL: https://www.wjgnet.com/1007-9327/full/v13/i16/2283.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i16.2283
Esophageal cancer is an aggressive disease, and the presence of lymph-node metastasis and vascular invasion indicate high malignant potential[1]. Surgery is the treatment of choice for patients with locoregionally confined esophageal cancer; however, the 5-year survival prognosis is less than 50%, even after curative surgery[2]. The development of improved prognostic markers and effective treatment strategies is therefore of great importance; this process depends on our understanding of the biological behavior of the tumors, and the identifying factors responsible for tumor invasiveness, metastasis, and recurrence.
The initiation, growth, and development of new blood vessels through angiogenesis are essential processes for tumor growth and dissemination. Inhibiting tumor blood-vessel formation[3,4] offers promising therapeutic approaches. In pathological angiogenesis, the angiogenic switch is shifted towards the proangiogenic factors, which results in irregular tumor vessel growth. Blood vessel growth in normal tissues is regulated through a delicate and complex equilibrium between the collective actions of proangiogenic factors and angiogenic inhibitors. Many factors are involved in regulating the equilibrium between angiogenic stimulants and inhibitors, including vascular endothelial growth factor (VEGF), methionine aminopeptidase-2 (MetAP-2), p53, tubulin, cyclooxygenase-2 (COX-2), and matrix metalloproteinases (MMPs).
VEGF is the best-characterized proangiogenic factor[5,6] and belongs to the platelet-derived growth factor superfamily. It potently increases vascular permeability, and promotes the formation of new blood vessels by stimulating endothelial cells to migrate and divide. VEGF is overexpressed in the majority of human solid cancers, where it is correlated with poor prognosis[7-10]. COX-2, which is an enzyme responsible for the formation of prostaglandin H2 from arachidonic acid, regulates angiogenesis, and COX-2-selective inhibitors can inhibit the process of colorectal tumorigenesis in both mice and humans[11-13]. COX-2 and VEGF expression are closely linked, and play critical roles in tumor progression and aggressiveness.
Since 1996, our institute has offered preoperative chemoradiotherapy (CRT) combined with radical surgery for the treatment of esophageal cancers. This has led to increased resectability, reduced incidence of both local recurrence and distant metastasis, and better prognosis for CRT responders[14]. However, the application of CRT remains controversial, and the prognosis of patients undergoing CRT has not been reliably estimated. In the current study, we retrospectively investigated the expression of COX-2 protein in human esophageal squamous cell carcinoma (ESCC) tissues, and evaluated the clinical implications for patients who underwent preoperative CRT and radical surgery.
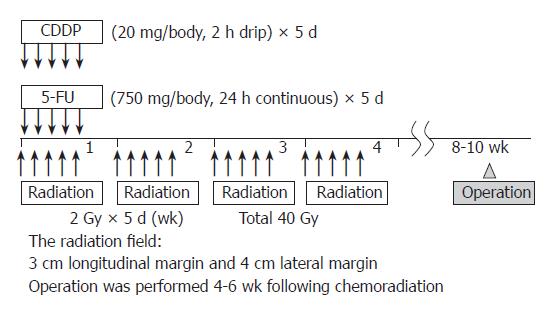
In total, 40 patients (nine women and 31 men; mean age, 60 years; age range, 44-78 years) with surgically excised ESCC were studied at the Hyogo College of Medicine, Japan, between April 1996 and December 2005. Preoperative CRT was performed as follows (Figure 1): 5-flurouracil (5-FU; 500 mg/m2 per day) was administered as a 120 h continuous intravenous (i.v.) infusion starting on d 1, and cisplatin (CDDP) (15 mg/m2 per day) was administered as a 2 h i.v. infusion on d 1-5. Radiation therapy was performed on d 1-5, after CDDP infusion, using a linear accelerator (Mevatron KD2, Siemens, Germany) and the previously described radiation method[14]. Chemotherapy was combined with radiation therapy during the first week, then radiation therapy alone was repeated for the next 3 wk (d 8-12, 15-19, and 22-26). Each single dose of radiation was 2 Gy/d, with a total dose of 40 Gy. Surgery was usually performed 4-6 wk after the completion of CRT. Resected specimens were cut open longitudinally and fixed with formalin. Follow-up information was obtained from office charts, hospital records, and telephone interviews. The ethics committee of the institution approved the study protocol.
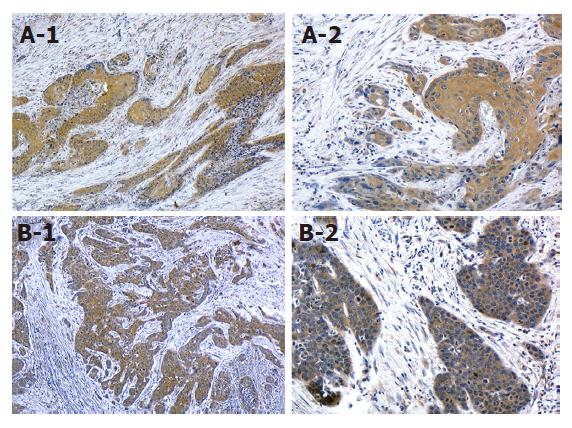
ESCC tissue specimens were processed using conventional procedures for paraffin embedding, cut into 4 μm sections, and mounted onto poly-l-lysine-coated slides. Sections were dewaxed in xylene and rehydrated in a descending series of alcohols. They were then blocked for endogenous peroxidase with 30 mL/L H2O2 in methanol, and blocked for non-specific antibody binding with normal rabbit serum. Sections were incubated for 2 h at room temperature with goat polyclonal anti-human COX-2 antibody and mouse monoclonal anti-human VEGF antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA), followed by a standard avidin-biotin-peroxidase complex method. The slides were developed with 3, 3’-diaminobenzidine tetrahydrochloride solution containing 1 mL/L H2O2 and were lightly counterstained with hematoxylin. Normal mouse immunoglobulin G (IgG) was substituted for the primary antibody as a negative control. The sections were finally counterstained with Lillie-Mayer’s hematoxylin, mounted, and examined microscopically by three of the authors (Y.F., K.K., and T.T.) without knowledge of clinicopathologic features. The results were graded on a scale of 0-3 based on the percentage of specific tumor-cell staining as follows: grade 0, no specific staining or < 5% of the tumor cells; grade 1, ≥ 5% to < 35% of the tumor cells; grade 2, ≥ 35% to < 65% of the tumor cells; grade 3, ≥ 65% tumor cells. Grades 0 and 1 were considered as low expression, and grades 2 and 3 were considered as high expression.
Overall survival (OS) was defined as the time from the date of initial diagnosis to patient death or the date of the last available information on vital status. Disease-free survival (DFS) was defined as the length of time after treatment during which no cancer was found. In univariate analysis, the difference between the cumulative survival rates of patient groups was calculated by the log-rank test for comparison using Kaplan-Meier survival curves. Statistical significance was considered at P < 0.05. Statistical analyses were carried out using Statistica software, version 06J (Statistica, Tulsa, OK, USA).
The patient group showed a male gender bias (male:female, 31:9). The histology of all tumors was shown to be ESCC. Thirty-six tumors originated in the thorax. According to the Tumor-node-metastasis (TNM) system of the American Joint Committee on Cancer, stage II tumors were seen in 15 patients (37.5%), stage III tumors were seen in 16 (40.0%), and stage IV tumors were seen in nine (22.5%). Seventeen patients (42.5%) had lymph-node metastasis at the time of diagnosis. All lesions before CRT presented with a T3 or T4 extent of invasion. Three-quarters of the patients had tumors between 6 and 8 cm in diameter. The M+ classification was given to six tumors. Two patients had distant metastasis of the liver. All patients experienced a disease-free period. During the follow-up period, five patients (12.5%) developed local recurrence or residual tumors, six (15.0%) developed neck or celiac lymph-node recurrence, and seven (17.5%) developed distant metastasis. Sixteen patients (40.0%) died during follow-up: 14 (35.0%) of these died from their tumors, whereas the remaining two (2.0%) were tumor free and died of intercurrent diseases (Table 1).
| Characteristics | n | |
| Sex (M/F) | 40 (31/9) | |
| Mean age (yr) | 60 | |
| Location of tumor | Cervical | 2 |
| Upper thoracic | 5 | |
| Middle thoracic | 22 | |
| Lower thoracic | 11 | |
| T-classification | T3 | 18 |
| T4 | 22 | |
| N-classification | N0 | 23 |
| N1 | 17 | |
| M-classification | M0 | 34 |
| M1 | 6 | |
| UICC TNM stage | IIa | 15 |
| III | 16 | |
| IVa | 5 | |
| IVb | 4 |
Fourteen tumors were eradicated by CRT, resulting in an absence of visible tumor cells described as a pathological complete response (CR). No COX-2 expression was detected in nine tumors, and VEGF expression was absent from 13 tumors (34.6% and 50.0%, respectively). Seventeen tumors (65.4%) were positive for COX-2 expression: 12 scored 1, five scored 2, and none scored 3. Thirteen tumors (50.0%) were positive for VEGF expression: five scored 1, six scored 2, and two scored 3. One of the two patients who scored 3 for VEGF expression died of multiple lung metastasis 2 months after surgery.
Positive COX-2 expression was shown to be related to VEGF expression (P < 0.05). Staining for both proteins was predominantly observed in the cytoplasm of tumor cells (Figure 2). In the COX-2/VEGF-positive expression group (n = 13), recurrences were found in nine patients: two locally and eight distant in the liver, bone, thyroid gland, lung, and neck lymph nodes. By contrast, in the null COX-2 or VEGF expression group (n = 13), recurrences were found in only two patients in the liver and bone.
After follow-up, disease progression was detected in nine of the 13 COX-2/VEGF-positive patients, four of whom died. A univariate analysis of the OS prognostic factors is summarized in Table 2. The results revealed that lymph-node metastasis and distant metastasis had significant prognostic value (P = 0.012 and P = 0.00026, respectively). Furthermore, there was a significant effect of CRT on OS prognosis (P = 0.0019).
| n | P | P | ||
| Covariate | for OS | for COX-2 | ||
| Age (yr) | < 70 | 34 | NS | NS |
| ≥ 70 | 6 | |||
| Gender | Male | 31 | NS | NS |
| Female | 9 | |||
| Effect of CRT | Effective | 32 | 0.0019b | NS |
| Ineffective | 8 | |||
| Lymph node metastasis | Positive | 17 | 0.012a | 0.0095b |
| Negative | 23 | |||
| Distant metastasis | Positive | 6 | 0.00026b | 0.0277a |
| Negative | 34 | |||
| Depth of tumor invasion | T3 | 18 | 0.0401a | NS |
| T4 | 22 | |||
| Tumor location1 | Upper | 7 | NS | NS |
| Lower | 33 | |||
| COX-2 expression | Positive | 17 | NS | - |
| Negative | 23 | |||
| VEGF expression | Positive | 13 | NS | - |
| Negative | 27 |
Kaplan-Meier analyses did not find a low OS for patients with persistent positive COX-2 or VEGF expression in their primary tumors, compared with null COX-2 or VEGF expression patients, or those with no residual tumor (Table 2). However, patients with null COX-2 or VEGF expression, or no residual tumors, showed an improved DFS compared with other patients (log-rank test; P = 0.0073 and P = 0.0341, respectively; Figures 3, and 4). Those with only null COX-2 expression showed no significantly improved DFS compared with those with COX-2 expression (log-rank test; P = 0.07).
Understanding the role of molecular factors in the acquisition of esophageal cancer malignant potential is important in treating these aggressive tumors. Recently, high COX-2 expression was shown to associate with increased intratumoral microvessel density and suppression of tumor cell apoptosis in human ESCCs[15,16]. Cells showing COX-2 overexpression tend to be resistant to undergo apoptosis[11]. Notably, the present study demonstrated that COX-2 expression was an influential factor for lymph-node metastasis and poorer prognosis in operable ESCC patients who underwent CRT. COX-2 signaling pathway is also related to tumor angiogenesis via its association with VEGF expression in ESCC and colorectal cancer[17,18]. Taken together, these findings suggest that ESCCs with high COX-2 expression might be less sensitive to CRT because of increased vascularization, and anti-apoptotic action against anticancer agents and radiation therapy.
Recently, high COX-2 expression following neoadjuvant CRT has been reported to be associated with tumor regression grade responding to CRT[19]. To date, few reports have examined the relationship between COX-2 and VEGF expression and clinicopathological parameters in ESCC patients who are undergoing preoperative CRT and surgical resection. Our results suggest that COX-2 expression after CRT might be a useful biomarker for the screening and management of high-risk patients with more frequent metastasis.
Overexpression of COX-2 has been reported in several human malignancies, including esophageal cancer[20]. Accumulating evidence indicates that selective COX-2 inhibitors show antitumor activity, and have synergistic effects with chemotherapy and/or radiotherapy. Some COX-2 inhibitors also reduce proliferation and increase apoptosis in esophageal cancer both in vitro and in vivo[21-24]. Thus, these inhibitors might have the potential to act as radiosensitizers due to increased intrinsic cell radiosensitivity and inhibition of tumor angiogenesis[25]. Consequently, the antitumor effect of COX-2 inhibitors is considered as at least partially antiangiogenetic in nature.
A recent study on lung adenocarcinoma samples suggested that COX-2 might be involved in the regu-lation of lymphangiogenesis via induction of the lymphangiogenetic factor VEGF-C[19]; similar effects were also suggested for ESCC[15]. Together with our results, these findings indicate that the COX-2/VEGF interaction is important for the metastasis of solid tumors that fail to respond to CRT, although the alteration of COX-2 and VEGF expression by CRT was not directly analyzed in our current study. Application of COX-2 inhibitors in combination with CRT should therefore be explored for ESCC patients.
In the present study, patients with null COX-2 expression or no residual tumors showed an improved DFS compared with other patients; however, there was no evidence for a significantly improved OS. The reason for this discrepancy remains to be clarified. The association between high COX-2 expression and limited patient response to chemotherapy, particularly platinum alone[26-28], is supported by our data, which confirm that adjuvant chemotherapy after surgery is more influential for the prognosis of ESCC patients than the status of COX-2 expression after CRT.
At our institution, a regimen of platinum and taxane (e.g. paclitaxel, docetaxel) is the preferred first-line adjuvant treatment for ESCC, which might reflect an improved OS in patients with COX-2 expression. Taxane interferes with microtubule formation, and its antiangiogenic effects have been shown to be partly due to its preferential accumulation in endothelial cells[29]. A modality utilizing taxane plus COX-2 inhibitor might be an attractive antiangiogenic and antitumor strategy in the adjuvant setting. Therefore, more attention should be required in choosing chemotherapeutic modality (including antiangiogenic agents in the near future) for the high-risk patients showing high levels of COX-2 expression.
In conclusion, we have demonstrated that positive COX-2 expression after CRT correlates with lymph-node metastasis and is a possible prognostic factor in ESCC progression-free survival. These findings suggest that COX-2 plays an important role in ESCC lymphangiogenesis, and is a promising molecular target for the treatment of ESCC. Large-cohort studies in a multicenter setting will be necessary to validate our findings, and to explore the use of taxane in postoperative adjuvant chemotherapy.
This report was presented in part at the 2007 American Society of Clinical Oncology Gastrointestinal Cancers Symposium, Orlando, FL, USA, 19th-21st, Jan, 2007.
Since 1996, our institute has offered preoperative chemoradiotherapy (CRT) combined with radical surgery for the treatment of esophageal cancers. This has led to increased resectability, reduced incidence of both local recurrence and distant metastasis, and better prognosis for CRT responders. However, the application of CRT remains controversial, and the prognosis of patients undergoing CRT has not been reliably estimated.
Recently, high COX-2 expression following neoadjuvant CRT has been reported to be associated with tumor regression grade responding to CRT. To date, few reports have examined the relationship between COX-2 and VEGF expression and clinicopathological parameters in esophageal squamous cell carcinoma (ESCC) patients who are undergoing preoperative CRT and surgical resection.
Our results suggest that COX-2 expression after CRT might be a useful biomarker for the screening and management of high-risk patients with more frequent metastasis, and that the antitumor effect of COX-2 inhibitors is considered as at least partially antiangiogenetic in nature.
Accumulating evidence indicates that selective COX-2 inhibitors show antitumor activity, and have synergistic effects with chemotherapy and/or radiotherapy. Our results suggest that combination therapy with these inhibitors might have the potential to inhibit recurrences.
VEGF is the best-characterized proangiogenic factor. It potently increases vascular permeability, and promotes the formation of new blood vessels by stimulating endothelial cells to migrate and divide. VEGF is overexpressed in the majority of human solid cancers, where it is correlated with poor prognosis. COX-2, which is an enzyme responsible for the formation of prostaglandin H2 from arachidonic acid, regulates angiogenesis, and COX-2-selective inhibitors can inhibit the process of colorectal tumorigenesis in both mice and humans.
The work is a retrospective immunohistochemical stainings of COX-2 and VEGF on resected esophageal cancer specimens from 40 patients undergone preoperative neoadjuvant chemoradiotherapy. The combination of staining COX-2 and VEGF is novel in esophageal squamous cell carcionoma, yet the scientific rationale need more solid explanation. The authors showed that COX-2 expression after preoperative CRT correlated with VEGF expression, lymph node metastasis, and recurrence, and is a possible prognostric factor in ESCC. Since this is a retrospective pathological specimen-based study, a clear understanding of the patient flow may help us in sound judgement and in preventing selection bias.
S- Editor Zhu LH L- Editor Wang XL E- Editor Che YB
| 1. | Sugimachi K, Matsuoka H, Ohno S, Mori M, Kuwano H. Multivariate approach for assessing the prognosis of clinical oesophageal carcinoma. Br J Surg. 1988;75:1115-1118. |
| 2. | Tachibana M, Kinugasa S, Yoshimura H, Shibakita M, Tonomoto Y, Dhar DK, Nagasue N. Clinical outcomes of extended esophagectomy with three-field lymph node dissection for esophageal squamous cell carcinoma. Am J Surg. 2005;189:98-109. |
| 3. | Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182-1186. |
| 4. | Folkman J. Anti-angiogenesis: new concept for therapy of solid tumors. Ann Surg. 1972;175:409-416. |
| 5. | Connolly DT, Heuvelman DM, Nelson R, Olander JV, Eppley BL, Delfino JJ, Siegel NR, Leimgruber RM, Feder J. Tumor vascular permeability factor stimulates endothelial cell growth and angiogenesis. J Clin Invest. 1989;84:1470-1478. |
| 6. | Keck PJ, Hauser SD, Krivi G, Sanzo K, Warren T, Feder J, Connolly DT. Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science. 1989;246:1309-1312. |
| 7. | Linderholm BK, Lindahl T, Holmberg L, Klaar S, Lennerstrand J, Henriksson R, Bergh J. The expression of vascular endothelial growth factor correlates with mutant p53 and poor prognosis in human breast cancer. Cancer Res. 2001;61:2256-2260. |
| 8. | Ishigami SI, Arii S, Furutani M, Niwano M, Harada T, Mizumoto M, Mori A, Onodera H, Imamura M. Predictive value of vascular endothelial growth factor (VEGF) in metastasis and prognosis of human colorectal cancer. Br J Cancer. 1998;78:1379-1384. |
| 9. | Fontanini G, Vignati S, Boldrini L, Chinè S, Silvestri V, Lucchi M, Mussi A, Angeletti CA, Bevilacqua G. Vascular endothelial growth factor is associated with neovascularization and influences progression of non-small cell lung carcinoma. Clin Cancer Res. 1997;3:861-865. |
| 10. | Inoue K, Ozeki Y, Suganuma T, Sugiura Y, Tanaka S. Vascular endothelial growth factor expression in primary esophageal squamous cell carcinoma. Association with angiogenesis and tumor progression. Cancer. 1997;79:206-213. |
| 11. | Tsujii M, Kawano S, Tsuji S, Sawaoka H, Hori M, DuBois RN. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell. 1998;93:705-716. |
| 12. | Oshima M, Dinchuk JE, Kargman SL, Oshima H, Hancock B, Kwong E, Trzaskos JM, Evans JF, Taketo MM. Suppression of intestinal polyposis in Apc delta716 knockout mice by inhibition of cyclooxygenase 2 (COX-2). Cell. 1996;87:803-809. |
| 13. | Arber N, Eagle CJ, Spicak J, Rácz I, Dite P, Hajer J, Zavoral M, Lechuga MJ, Gerletti P, Tang J. Celecoxib for the prevention of colorectal adenomatous polyps. N Engl J Med. 2006;355:885-895. |
| 14. | Fujiwara Y, Kamikonya N, Inoue T, Koishi K, Yoshikawa R, Nakao K, Yagyu R, Nishiwaki M, Fujiwara M, Kojima S. Chemoradiotherapy for T3 and T4 squamous cell carcinoma of the esophagus using low-dose FP and radiation: a preliminary report. Oncol Rep. 2005;14:1177-1182. |
| 15. | Kase S, Osaki M, Honjo S, Adachi H, Tsujitani S, Kaibara N, Ito H. Expression of cyclo-oxygenase-2 is correlated with high intratumoral microvessel density and low apoptotic index in human esophageal squamous cell carcinomas. Virchows Arch. 2003;442:129-135. |
| 16. | Hironaka S, Hasebe T, Kamijo T, Ohtsu A, Boku N, Yoshida S, Saitoh H, Ochiai A. Biopsy specimen microvessel density is a useful prognostic marker in patients with T(2-4)M(0) esophageal cancer treated with chemoradiotherapy. Clin Cancer Res. 2002;8:124-130. |
| 17. | Cianchi F, Cortesini C, Bechi P, Fantappiè O, Messerini L, Vannacci A, Sardi I, Baroni G, Boddi V, Mazzanti R. Up-regulation of cyclooxygenase 2 gene expression correlates with tumor angiogenesis in human colorectal cancer. Gastroenterology. 2001;121:1339-1347. |
| 18. | von Rahden BH, Stein HJ, Pühringer F, Koch I, Langer R, Piontek G, Siewert JR, Höfler H, Sarbia M. Coexpression of cyclooxygenases (COX-1, COX-2) and vascular endothelial growth factors (VEGF-A, VEGF-C) in esophageal adenocarcinoma. Cancer Res. 2005;65:5038-5044. |
| 19. | Xi H, Baldus SE, Warnecke-Eberz U, Brabender J, Neiss S, Metzger R, Ling FC, Dienes HP, Bollschweiler E, Moenig S. High cyclooxygenase-2 expression following neoadjuvant radiochemotherapy is associated with minor histopathologic response and poor prognosis in esophageal cancer. Clin Cancer Res. 2005;11:8341-8347. |
| 20. | Zimmermann KC, Sarbia M, Weber AA, Borchard F, Gabbert HE, Schrör K. Cyclooxygenase-2 expression in human esophageal carcinoma. Cancer Res. 1999;59:198-204. |
| 21. | Souza RF, Shewmake K, Beer DG, Cryer B, Spechler SJ. Selective inhibition of cyclooxygenase-2 suppresses growth and induces apoptosis in human esophageal adenocarcinoma cells. Cancer Res. 2000;60:5767-5772. |
| 22. | Petersen C, Petersen S, Milas L, Lang FF, Tofilon PJ. Enhancement of intrinsic tumor cell radiosensitivity induced by a selective cyclooxygenase-2 inhibitor. Clin Cancer Res. 2000;6:2513-2520. |
| 23. | Kishi K, Petersen S, Petersen C, Hunter N, Mason K, Masferrer JL, Tofilon PJ, Milas L. Preferential enhancement of tumor radioresponse by a cyclooxygenase-2 inhibitor. Cancer Res. 2000;60:1326-1331. |
| 24. | Pyo H, Choy H, Amorino GP, Kim JS, Cao Q, Hercules SK, DuBois RN. A selective cyclooxygenase-2 inhibitor, NS-398, enhances the effect of radiation in vitro and in vivo preferentially on the cells that express cyclooxygenase-2. Clin Cancer Res. 2001;7:2998-3005. |
| 25. | Milas L, Kishi K, Hunter N, Mason K, Masferrer JL, Tofilon PJ. Enhancement of tumor response to gamma-radiation by an inhibitor of cyclooxygenase-2 enzyme. J Natl Cancer Inst. 1999;91:1501-1504. |
| 26. | Ferrandina G, Lauriola L, Distefano MG, Zannoni GF, Gessi M, Legge F, Maggiano N, Mancuso S, Capelli A, Scambia G. Increased cyclooxygenase-2 expression is associated with chemotherapy resistance and poor survival in cervical cancer patients. J Clin Oncol. 2002;20:973-981. |
| 27. | Raspollini MR, Amunni G, Villanucci A, Boddi V, Baroni G, Taddei A, Taddei GL. Expression of inducible nitric oxide synthase and cyclooxygenase-2 in ovarian cancer: correlation with clinical outcome. Gynecol Oncol. 2004;92:806-812. |
| 28. | Ferrandina G, Ranelletti FO, Martinelli E, Paglia A, Zannoni GF, Scambia G. Cyclo-oxygenase-2 (Cox-2) expression and resistance to platinum versus platinum/paclitaxel containing chemotherapy in advanced ovarian cancer. BMC Cancer. 2006;6:182. |
| 29. | Merchan JR, Jayaram DR, Supko JG, He X, Bubley GJ, Sukhatme VP. Increased endothelial uptake of paclitaxel as a potential mechanism for its antiangiogenic effects: potentiation by Cox-2 inhibition. Int J Cancer. 2005;113:490-498. |