Published online Apr 21, 2007. doi: 10.3748/wjg.v13.i15.2160
Revised: December 26, 2006
Accepted: January 26, 2007
Published online: April 21, 2007
AIM: To evaluate a newly developed hand-held confocal probe for in vivo microscopic imaging of the complete gastrointestinal tract in rodents.
METHODS: A novel rigid confocal probe (diameter 7 mm) was designed with optical features similar to the flexible endomicroscopy system for use in humans using a 488 nm single line laser for fluorophore excitation. Light emission was detected at 505 to 750 nm. The field of view was 475 μm × 475 μm. Optical slice thickness was 7 μm with a lateral resolution of 0.7 μm. Subsurface serial images at different depths (surface to 250 μm) were generated in real time at 1024 × 1024 pixels (0.8 frames/s) by placing the probe onto the tissue in gentle, stable contact. Tissue specimens were sampled for histopathological correlation.
RESULTS: The esophagus, stomach, small and large intestine and meso, liver, pancreas and gall bladder were visualised in vivo at high resolution in n = 48 mice. Real time microscopic imaging with the confocal mini-microscopy probe was easy to achieve. The different staining protocols (fluorescein, acriflavine, FITC-labelled dextran and L. esculentum lectin) each highlighted specific aspects of the tissue, and in vivo imaging correlated excellently with conventional histology. In vivo blood flow monitoring added a functional quality to morphologic imaging.
CONCLUSION: Confocal microscopy is feasible in vivo allowing the visualisation of the complete GI tract at high resolution even of subsurface tissue structures. The new confocal probe design evaluated in this study is compatible with laparoscopy and significantly expands the field of possible applications to intra-abdominal organs. It allows immediate testing of new in vivo staining and application options and therefore permits rapid transfer from animal studies to clinical use in patients.
-
Citation: Goetz M, Memadathil B, Biesterfeld S, Schneider C, Gregor S, Galle PR, Neurath MF, Kiesslich R.
In vivo subsurface morphological and functional cellular and subcellular imaging of the gastrointestinal tract with confocal mini-microscopy. World J Gastroenterol 2007; 13(15): 2160-2165 - URL: https://www.wjgnet.com/1007-9327/full/v13/i15/2160.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i15.2160
Rapid microscopic diagnosis is essential for the successful management of most diseases of the gastrointestinal (GI) tract. Ex vivo histological examination is currently the gold standard for definite tissue diagnosis, but is associated with potential drawbacks. Processing of the specimens is time consuming and may delay definite diagnosis, and invasive tissue sampling carries the risk of bleeding and infection and is prone to sampling error.
Laser scanning confocal microscopy is an adaptation of white light microscopy in which tissue structures are visualized by stimulating emission of light by a low power laser beam after application of fluorescent contrast agents[1]. The intensity of emitted light is translated into a greyscale image which represents microscopic tissue structures. Since confocal pinholes geometrically reject out of focus light, high resolution imaging even of subsurface structures is feasible. In vivo application of confocal microscopy has been impaired by bulky bench top devices. In those, specimens were required to be fixed relative to the microscope lens, limiting imaging possibilities especially in live animals or even humans. Therefore, much effort has been put into in vivo applicability of imaging devices[2-4]. Recently, miniaturization has allowed for the integration of a confocal scanning head into a colonoscope, thus permitting application for in vivo imaging in humans during ongoing endoscopy and discrimination of normal and neoplastic tissue with high accuracy[5-7]. However at current only a limited number of fluorescent dyes has been registered for clinical use in humans. This limits the (patho-) physiological issues that can be addressed in vivo. In addition, only those regions of the GI tract that are accessible by flexible endoscopy could be examined. Technological advances have resulted in the integration of a further miniaturized confocal scanning head into a rigid probe with an outer diameter of 7 mm. The aim of the current study was to evaluate this new imaging technique for the first time for in vivo visualization of the complete GI tract in rodents at real time with multiple staining techniques for subsurface confocal morphological and functional imaging.
In the miniaturized confocal microscopy probe (prototype FIVE1, Optiscan, Australia), the x-y-scan mechanism and the z-axis-actuator have been integrated into the distal tip of the probe with an outer diameter of 7 mm that could be used hand-held in a manner comparable to holding a pen. The probe was flexibly connected to the laser source and the detection unit. A solid state laser delivered an excitation wavelength of 488 nm, and light emission was detected at 505 to 585 nm. Actuation of the imaging plane depth along the range of the z-axis (surface to 250 μm) was achieved by means of an electrically actuated shape memory alloy which moved the scanner relative to the imaging window and was controlled using two remote control buttons on a foot pedal. Laser power output at the tissue surface could be adjusted during imaging from 0 to 1000 μW to achieve appropriate tissue contrast. Each resultant image was a transverse optical section of 500 μm × 500 μm, representing approximately one focal plane within the specimen. Serial images were collected at a scan rate of 0.8 frame/s at 1024 × 1024 pixels or 1.6 frames/s at 1024 × 512 pixels, approximating a 1 000 fold magnification on a 19 inch screen. Mono-colouring of images was performed in a single-step procedure, and three-dimensional tissue models reconstructed from z-stacks (both with ImageJ 1.30 v, W. Rasband, National Institutes of Health, USA).
For confocal imaging, n = 48 healthy mice (male or female, C57BL/6 and FVB) were deeply anaesthetized using 300 μL avertin i.p. (1 g of 2, 2, 2-tribromo-ethanol per 1 mL tertiary amyl alcohol (both Sigma-Aldrich, Germany), 0.25 mL in 10 mL phosphate buffered saline). The intra-abdominal organs were identified after a small median laparotomy. The bowel was first examined from the external side, and afterwards opened on the anti-mesenteric side to maintain bowel perfusion during imaging. Bleeding was prevented by cauterisation. The bowel mucosa was exposed and flushed with 0.9% saline. The esophagus was partially mobilised through the diaphragm or visualised after thoracotomy. The probe was directly placed onto the tissue by using the probe hand-held or mounting it onto a stereotactic frame, as appropriate to minimize motion artefacts. An in-built contact window provided the function of a cover slip. Concentration and total volume of fluorescent agents and time of exposure (in topical use of acriflavine) were optimized by immediate evaluation of the collected images and adaptation of laser power output to tissue contrast or by re-staining, if necessary. Per examination, 50 to 500 images were collected with the help of a foot pedal and digitally stored as greyscale images. After in vivo imaging, animals were sacrificed by an avertin overdose and tissue specimens collected for histopathological correlation. Animal procedures were approved by the local ethical committee and were in accordance with national and international guidelines.
Confocal images were collected after bolus intravenous or intracardial application of fluorescent agents; only acriflavine was also used for topical staining. Fluorescein sodium (1 g/10 mL, Alcon Pharma, Freiburg, Germany) was injected at 10 μL/g body weight. FITC-labelled dextran (25 mg/mL w/v in A. dest., MW 150 kDa, Sigma-Aldrich, Steinheim, Germany) was administered at 250 μg/g. For topical staining with acriflavine (Sigma Pharmaceuticals, Victoria, Australia), several drops of a 0.02% solution in saline were applied to the tissue surface. Excess dye was washed off with phosphate-buffered saline. For systemic application, 10 μL/g of a 0.1% acriflavine solution was injected. FITC-labeled Lycopersicon esculentum lectin (Vector, Burlingame, USA) was injected intravenously at 15 μg/g.
Tissue specimens were collected from the area examined in vivo by confocal microscopy, fixed in 2.5 g/L buffered formaldehyd, and embedded in paraffin. To facilitate correlation with confocal images both vertical and transverse serial sections (4 μm) were obtained and stained with hematoxylin & eosin. After in vivo application of L. esculentum lectin, additional specimens were snap-frozen in liquid nitrogen and stored at -80°C, until further processed. Benchtop confocal microscopy (Zeiss, Jena, Germany) of cryosections was performed according to standard procedures. FITC-lectin restaining was not necessary, Hoechst 33342 staining (Molecular Probes, Burlingame, USA) was used for ex vivo nuclear contrast, as previously described[8].
The different staining protocols used in this study permitted almost immediate high resolution microscopic imaging in vivo. No immediate toxicity was observed after topical or systemic application. Fluorescein sodium that has been safely used in ophthalmology in humans for decades gave a good overall impression of the parenchyma and connective tissue architecture after partly leaking from the circulation into the tissue. It rendered many subcellular details such as mucin in colonic goblet cells. A sufficient contrast was also observed in the vessels where fluorescein was partly retained because of its plasma protein binding. Due to its pharmacological properties, fluorescein did not stain nuclei. This could be achieved by the application of acriflavine, which interacts with nucleic acids in the cell nucleus, but also stained the cytoplasm to a sufficient extent to permit tissue characterization. With this staining technique, nuclear details such as nucleolus formation in tissues with high transcriptional activity, e.g. liver, could also be visualized. Acriflavine also yielded high resolution images after topical application, indicating a use in diagnostic procedures in humans. FITC-labelled dextran was retained in the intact circulation due to its high molecular weight to a large extent. Thus, vessels could not only be visualized on a morphologic basis, but also perfusion of different organs could be monitored over a long time period, adding a time- and motion-related quality to morphologic imaging. This functional aspect is often only incomprehensively rendered by conventional ex vivo histology. After intravenous injection of FITC-labelled L. esculentum lectin, vessel walls were highlighted by selective targeting of glycoprotein moieties on endothelial cells. This yielded a three-dimensional impression of the capillary system when scanning through the mucosa along the whole range of the z-axis. Photobleaching and washing out of fluorescent agents started 20 min after injection. However, examination times up to 60 min with good quality were achieved. No parenchymal damage by the laser light was noted macroscopically or histopathologically.
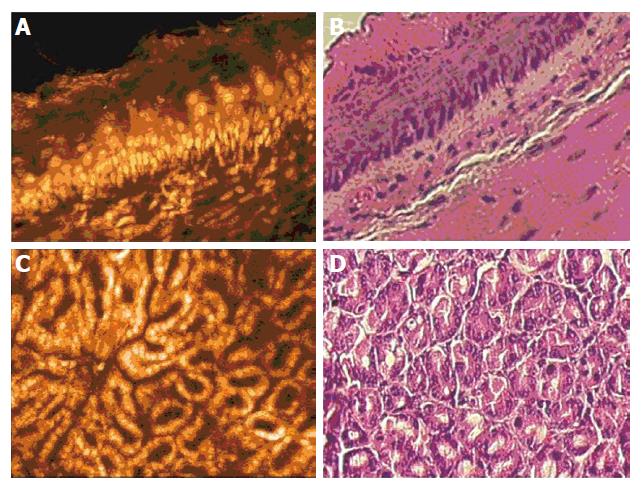
In the esophagus, application of acriflavine gave a clear overview of the structure of the mucosa when caught at an oblique angle (Figure 1A). Confocal imaging rendered a clearly stratified squamous epithelium with basal cells of the germinative zone sitting atop a basal membrane and smaller cells towards the luminal surface. Oval shaped nuclei identified endothelial cells, indicating vessels in the upper lamina propria. Correlation with ex vivo H&E staining was excellent (Figure 1B). In deeper sections, the vessels and endothelial cells of the lamina propria were visible. Movement artefacts caused by the animal’s breathing and heartbeat were only a problem in organs with direct contact to the diaphragm such as the esophagus, but even here more than 60 percent of the obtained images were of sufficient quality.
In the stomach, application of the mini-microscopy probe onto the tissue surface in an en face view successively rendered the gastric foveolae and glands when scanning through the mucosa. Acriflavine staining revealed the regular surface lining of the foveolae with columnar epithelial cells with basally oriented nuclei (Figure 1C). At levels close to the surface, the branched structure of the foveolae could be clearly seen, while in deeper tissue sections the tightly packed gastric glands had a round, crypt-like appearance. The different ratio of foveolar to glandular depth in the fundus, corpus and antrum could be semi-quantitatively assessed and correlated well with conventional histology (Figure 1D).
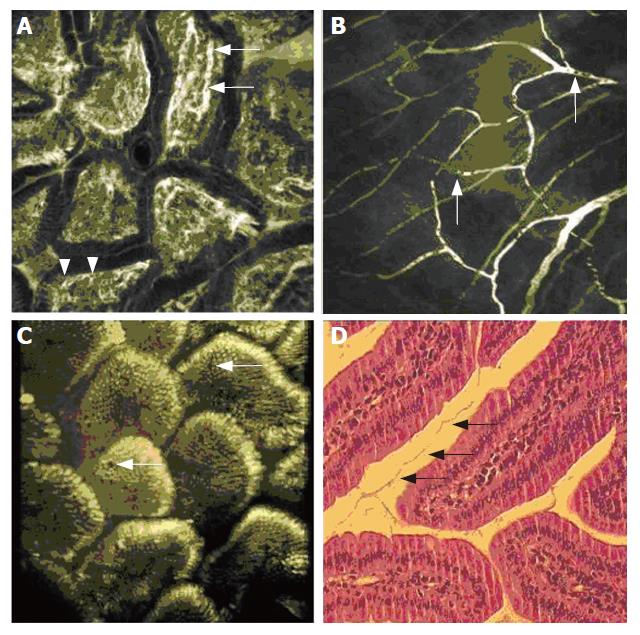
In the duodenum, villi were easily visualized with all staining protocols in vivo. Application of FITC-labelled dextran gave an excellent impression of the hairpin-like capillary network within each single villus (Figure 2A). Capillaries were displayed along the longitudinal villus axis in the loose connective tissue of the lamina propria just below the epithelial lining. Blood cells were rendered as a negative contrast within the capillaries (arrow). While immediately after injection of FITC-labelled dextran only vessels were contrasted, extravasation after 10 min of small amounts of contrast dye gave a sufficient contrast in the lamina propria, allowing for a clear distinction of the lamina propria from the columnar appearance of the enterocytes, separated by a basal membrane. No nuclei were visualized with FITC-labelled dextran or fluorescein, but sub cellular details such as the brush border of the villus enterocytes were recorded after fluorescein injection.
After injection of FITC-labelled dextran, also the blood flow of the capillaries within the small bowel meso could be visualised in vivo. Capillary diameter was the size of a single erythrocyte, displayed as a black spot in the brightly stained plasma (Figure 2B). The trafficking of blood cells in the capillaries could be followed in vivo.
In the mouse ileum, the villus structure was similarly visualized as in the upper GI-tract. Topical acriflavine application readily rendered the epithelial lining of the ileal villi (Figure 2C). Mucin inclusion in goblet cells did not contrast brightly with acriflavine, permitting easy distinction of goblet cells from enterocytes. Cells were visualized both in an en face view at the villus surface and in a transverse optical section at the villus verge. In vivo imaging showed a close assembly of villi while in the histological examination retraction of the villi upon fixation was noted (Figure 2D). A series of transverse optical sections at different imaging depths captured successively after topical acriflavine application was used for a three-dimensional reconstruction of the ileal mucosa. This is rendered in Suppl. Figure 1 as a video file.
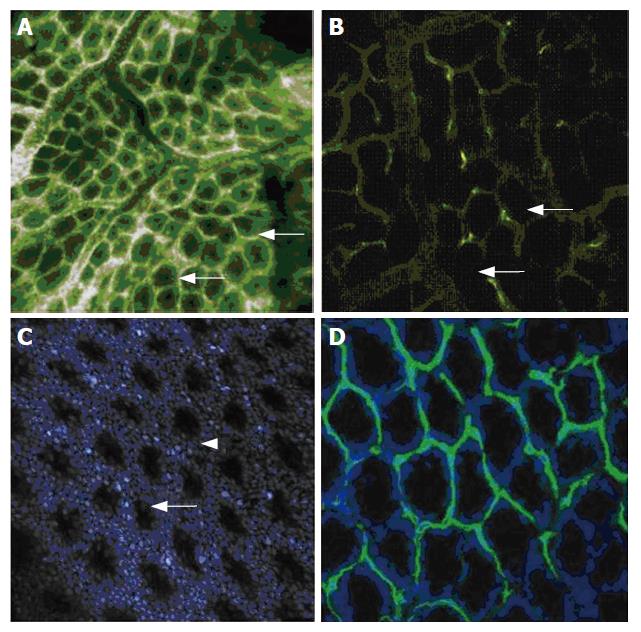
In the mouse colon, fluorescein sodium only highlighted vessels in the first seconds after injection, similar to FITC-labelled dextran. Fluorescein then distributed within the tissue, giving a good contrast of the tissue architecture and connecting tissue, while the dye was also partly retained within the circulation and permitted ongoing visualization of vessels. Sub cellular details such as mucin in goblet cells were noted close to the luminal surface. Resolution of sub cellular details was weaker when exploiting the full subsurface sectioning capability of the confocal probe. In the lamina propria of the colon, capillaries regularly surrounded each individual crypt in a honeycomb pattern. By visualization of deeper parts of the lamina propria, larger feeding and draining vessels were also seen (Figure 3A). A more selective staining of the capillary system of the colonic wall was achieved by injection of FITC-labelled dextran similar to imaging in the duodenum, but surrounding tissue was still contrasted due to dye extravasation. A selective staining of vessel walls of the colonic wall was achieved by using FITC-labelled L. esculentum lectin (Figure 3B). Blood plasma was not contrasted by this staining protocol. Cell nuclei were stained by topical or systemic acriflavine application (Figure 3C). Crypts were easily identified within the regular array of epithelial cells. The in vivo microscopic and functional images of the capillary system and blood flow were only insufficiently rendered by conventional H&E staining. After in vivo application of FITC-labelled L. esculentum lectin and ex vivo nuclear counter stain, cryosections of a snap-frozen colonic specimen yielded an overlay rendering both colonic cells and vessels when visualized with a conventional bench top confocal microscope (Figure 3D).
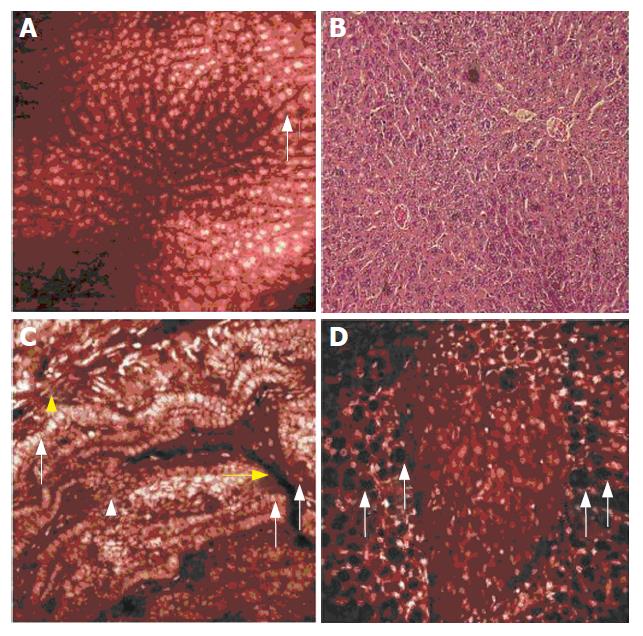
In mouse liver, intravenous application of acriflavine highlighted hepatocyte nuclei. With a lighter contrast in the cytoplasm and no contrast in sinusoids, liver cell plates could be clearly identified in their columnar arrangement, visualizing the lobular pattern of the liver in vivo (Figure 4A). Endothelial cells were visible at levels close to the tissue surface. Distinct granular patterns were demonstrated within the hepatocyte nuclei by confocal in vivo microscopy, corresponding to local accumulation of nucleic acid (nucleoli), and were confirmed in ex vivo H&E staining (Figure 4B).
Gall bladder visualization was best achieved with acriflavine (Figure 4C). A single layer of tall epithelial cells was clearly visualized on the numerous folds at the luminal side of the gall bladder wall. Acriflavine did not contrast the blood plasma, but vessels of the gall bladder wall were easily identified by their endothelial cell lining within the fibrovascular lamina propria. At levels closer to the extraluminal surface of the gall bladder, longitudinal and circular smooth muscle cells were easily differentiated by their typical arrangement.
In the mouse pancreas, patches of the gland were visualized within the intra-abdominal fatty tissue. Confocal imaging after acriflavine injection clearly identified the parenchyma, while the fatty tissue was characterized by its intracellular fat accumulation that was displayed as dark inclusions of slightly varying size within the adipocytes (Figure 4D).
A non-invasive imaging technique is highly desirable in almost any field in clinical medicine and basic science. In the present study, a newly developed hand-held confocal mini-microscopy probe allowed in vivo real time imaging of the complete gastrointestinal tract. This device permitted high resolution in vivo histological subsurface imaging during ongoing examination. The confocal probe used in this study shares many optical features with the flexible endomicroscopy system (Pentax EC-3870CIFK) registered for clinical use in humans, in which a miniaturized laser scanning head has been integrated into the distal tip of a videocolonoscope[5,7]. But at present application in patients suffers several limitations. First, only organs that are readily accessible by flexible endoscopy could be visualized so far. Second, for safety reasons only a limited range of fluorescent dyes have been registered for clinical use in patients so far. Third, examination times should be limited to avoid complications or patient discomfort during conscious sedation.
Animal studies do not suffer from these limitations. They offer a wide range of intravital staining and application options. The data presented here demonstrate possible applications of a further miniaturized hand-held confocal microscopy probe which represent an extension of the imaging capabilities of confocal in vivo microscopy. This device has been designed to be handled like a laparoscopy probe, and imaging was easy to perform by putting the confocal imaging window in close and stable contact with the organ of interest. No major adjustments were necessary during image acquisition. With the different staining techniques used in our study in mice, different aspects of both tissue morphology and function were highlighted. While many features of conventional histology are not yet available for in vivo microscopic imaging, the latter does not rely on fixation if applied by the new probe. Therefore, microscopic images are immediately available, allowing optical biopsy, vessels and blood flow can be rendered, and shrinking artifacts do not occur.
With this new probe design, confocal imaging is now easy to apply to any accessible organ. This allows high resolution in vivo imaging, with broad potential applications in different faculties of clinical medicine. Such applications could include, but are certainly not limited to early detection of cancer, for example of the oral cavity or the uterine cervix. By a laparoscopic approach determination of pathologic states such as inflammation and fibrosis of the liver, gall bladder or any other intra-abdominal organ could potentially be achieved by obtaining multiple optical biopsies without the need for invasive tissue sampling. Evaluation of completeness of tumor resection could be done at real time during ongoing surgery. The mouse models and probe used here will also allow rapid testing of staining procedures that carry potential for use in flexible endomicroscopy in patients, many of them already available for bench top confocal microscopy. This might accelerate the future transfer of in vivo imaging from bench to bedside. Confocal microscopy also permits molecular imaging in vivo, and recently fluorescent targeting and in vivo visualization of malignant cells has been described[9]. In addition, this new imaging modality also offers a broad spectrum of laboratory applications. It permits easy imaging of organs and cells in their natural environment without the need for a stationary and bulky microscopy stage. This is of paramount importance in different animal models of human diseases. These models are not limited to diseases of the gastrointestinal tract; in vivo visualization of microscopic details in almost any other organ is possible with high resolution[10].
In conclusion, in vivo confocal imaging of the complete gastrointestinal tract is feasible in rodents with a new hand-held mini-microscopy probe that allows high resolution real time subsurface imaging. Transfer of this technology and experience into patients will potentially further promote microscopic visualisation of human diseases in vivo of the whole gastrointestinal tract.
Ex vivo histological examination is currently the gold standard for definite tissue diagnosis, but is associated with potential drawbacks. Processing of the specimens is time consuming and may delay definite diagnosis and treatment, and invasive tissue sampling carries the risk of bleeding and infection and is prone to sampling error. Therefore, much effort has recently been put into the miniaturization of imaging devices to allow for immediate microscopic evaluation of tissue in vivo.
In vivo application of confocal microscopy to humans and animals has been limited by bulky bench-top devices requiring the organ of interest to be precisely fixed relative to a microscope stage. Recently, confocal endomicroscopy has been shown to reliably predict histology during ongoing endoscopy. However, only organs that are accessible by flexible endoscopy could be examined. Aim of the present study was to evaluate a newly developed hand-held confocal probe for in vivo microscopic imaging of the complete gastrointestinal tract in rodents.
The confocal microscopy probe evaluated in this study is miniaturized in a way to allow easy subsurface imaging with high magnification (up to 1000 fold) and high resolution, and at the same time, to be used in living animals (and humans) hand-held as a pen-like laparoscopy probe with an outer diameter of only 7 mm. Confocal microscopic images are readily available by simply putting the probe onto the tissue after administration of fluorescent dyes. The staining protocols used in this study permit highlighting of morphology and perfusion of the complete gastrointestinal tract on a microscopic level in vivo.
The confocal system used here has been applied to humans in flexible endoscopy. The current study shows the extensions of the application to organs that cannot be reached with flexible endomicroscopy and to staining protocols that are not yet registered for clinical use in humans. By using living animals for imaging and a laparoscopy-like imaging approach, rapid transfer of confocal imaging with this newly developed probe to patients is conceivable for rapid in vivo microscopic diagnosis in many fields of medicine.
Confocal microscopy: In confocal microscopy, the use of pinholes geometrically eliminates out of focus light. Therefore, resolution is enhanced especially in the axial direction as compared to white-light microscopy, allowing high resolution subsurface imaging.
It is a good and informative paper. The authors use a miniaturized probe to perform confocal microscopy in situ. They give the right arguments for using this probe.
S- Editor Wang J L- Editor Li M E- Editor Che YB
| 1. | Wright SJ, Wright DJ. Introduction to confocal microscopy. Methods Cell Biol. 2002;70:81-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Delaney P, Harris MR. Fiberoptics in confocal microscopy. Handbook of biological confocal microscopy. New York: Plenum Press 1995; 515-523. [DOI] [Full Text] |
| 3. | Sokolov K, Aaron J, Hsu B, Nida D, Gillenwater A, Follen M, MacAulay C, Adler-Storthz K, Korgel B, Descour M. Optical systems for in vivo molecular imaging of cancer. Technol Cancer Res Treat. 2003;2:491-504. [PubMed] |
| 4. | Flusberg BA, Cocker ED, Piyawattanametha W, Jung JC, Cheung EL, Schnitzer MJ. Fiber-optic fluorescence imaging. Nat Methods. 2005;2:941-950. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 616] [Cited by in RCA: 412] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 5. | Kiesslich R, Burg J, Vieth M, Gnaendiger J, Enders M, Delaney P, Polglase A, McLaren W, Janell D, Thomas S. Confocal laser endoscopy for diagnosing intraepithelial neoplasias and colorectal cancer in vivo. Gastroenterology. 2004;127:706-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 560] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 6. | Nathanson MH. Confocal colonoscopy: more than skin deep. Gastroenterology. 2004;127:987-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Polglase AL, McLaren WJ, Skinner SA, Kiesslich R, Neurath MF, Delaney PM. A fluorescence confocal endomicroscope for in vivo microscopy of the upper- and the lower-GI tract. Gastrointest Endosc. 2005;62:686-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 267] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 8. | Becker C, Fantini MC, Schramm C, Lehr HA, Wirtz S, Nikolaev A, Burg J, Strand S, Kiesslich R, Huber S. TGF-beta suppresses tumor progression in colon cancer by inhibition of IL-6 trans-signaling. Immunity. 2004;21:491-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 530] [Cited by in RCA: 589] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 9. | Goetz M, Fottner C, Schirrmacher E, Delaney P, Gregor S, Schneider C, Strand D, Kanzler S, Memadathil B, Weyand E. In-vivo confocal real-time mini-microscopy in animal models of human inflammatory and neoplastic diseases. Endoscopy. 2007;39:350-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |