Published online Apr 14, 2007. doi: 10.3748/wjg.v13.i14.2113
Revised: January 4, 2007
Accepted: January 14, 2007
Published online: April 14, 2007
AIM: To study the effect of adenovirus (Ad)-p53 gene therapy on hepatocellular carcinoma (HCC) in a rabbit model.
METHODS: VX2 tumor was grown in the liver of 24 rabbits. Animals were divided into four groups: group A receiving trans-arterial gene therapy (Ad-p53) only, group B receiving combined Ad-p53 therapy and trans-arterial embolization (lipiodol), group C receiving trans-arterial chemoembolization (lipiodol + mitomycin C), control group (D) receiving sodium chloride. Tumor volume (V1) was measured by using MRI (d 13). Interventional procedure was applied (d 14).Tumor volume (V2) was assessed by MRI (d 21) and the mean ratio (V2/V1) was calculated. After the second MRI, specimens of the liver were abstained and examined immunohistochemically using mutant-type p53 antibody. The positive expression was scored.
RESULTS: Compared with control group (= 3.14 ± 0.64), therapeutic groups all showed a significant decrease in the tumor growth ratio (P < 0.05) . A slight difference was found between group A (=2.35 ± 0.59) and group B (= 1.75 ± 0.28) (P = 0.048). No statistically significant difference was observed between group B and group C (= 2.00 ± 0.44). The positive expression rate of mutant-type p53 was the lowest in group B and significantly different between group A and group C (P < 0.05).Compared to the control subjects, groups A and C both showed a decrease in the expression of mutant-type p53, but there was no significant difference between them.
CONCLUSION: Trans-arterial Ad-p53 gene therapy can reduce tumor growth of HCC in rabbit model.
- Citation: Gu T, Li CX, Feng Y, Wang Q, Li CH, Li CF. Trans-arterial gene therapy for hepatocellular carcinoma in a rabbit model. World J Gastroenterol 2007; 13(14): 2113-2117
- URL: https://www.wjgnet.com/1007-9327/full/v13/i14/2113.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i14.2113
Hepatocellular carcinoma (HCC) is one of the most common malignancies worldwide, responsible for an estimated 1 million deaths annually and a poor prognosis due to its rapid infiltrating growth and complicating liver cirrhosis[1]. To date, surgical approaches including liver resection and liver transplantation are regarded potentially as curative treatments for HCC, particularly in patients with small and non-invasive tumors[2]. However, only a small number of patients are suitable for surgical treatment due to multicentric tumors, extrahepatic metastases, early vascular invasion, shortage of donor organs, high complication rates and comorbidities[3-5].
Local methods of tumor ablation including trans-arterial chemoembolization (TACE), percutaneous ethanol injection (PEI), radiofrequency ablation (RFA)[6-8], microwave coagulation therapy (MCT) and laser-induced thermotherapy (LITT), are promising additional tumor therapies, especially in patients with poor liver function and unresectable or multifocal tumors[9]. Since TACE was introduced as a palliative treatment in patients with unresectable HCC, it has become one of the most common forms of interventional proceduces[10-12]. TACE has been shown to reduce systemic toxicity, enhance local effects and thus improve the therapeutic results[11]. Its therapeutic effect is limited due to the lack of appropriate and reliable embolic agents in patients when the tumor is infiltrative in nature or hypovascular[13].
In the past years, it was proved that p53 gene absence is correlated with the occurrence of HCC[14]. Wild-type p53 tumor suppressor gene is praised as a gene guardian and the loss of wild-type p53 is thought to be responsible for the lack of apoptotic signals in tumor cells and thus for their uncontrolled proliferation and recurrence[15]. Many human tumors carry mutations in the p53 and mutant or absent p53 status has been associated with resistance to radiation therapy and to apoptosis-inducing chemotherapy[16]. Gendicine, a recombinant human Ad-p53 injection (Shenzhen Sibiono Bentech, China) has obtained a drug license from the State Food and Drug Administration of China (SFDA; Beijing, China) and become the first commercially-licensed gene therapy drug in the world. Gendicine consists of advenovirus vectors and normal wild-type p53 tumor suppressor gene. Gendicine was used in clinical trial on patients with late-stage head and neck squamous cell carcinoma (HNSCC), showing that complete and partial regression of the tumor can be achieved in 64% and 32% of patients, respectively[17]. A similar study by Sze et al[18] also showed that hepatic arterial infusion of p53 gene in combination with chemotherapy results in tumor regression. The purpose of our study was to compare the effects of the gene therapy and other therapies on HCC in rabbit model.
A total of 30 adult New Zealand white rabbits (3.0-3.5 kg) were used. The study was approved by the Animal Care Committee of Shandong University School of Medicine, and experiments were performed in accordance with the institutional guidelines. VX2 tumors were first grown for 2 wk on the hind legs of each of six carrier rabbits. Each carrier rabbit was used to supply tumor cells for implantation in four separate rabbits. The carrier rabbit was then killed under deep anesthesia by intravenous injection of 100 mg/kg pentobarbital. All animals were anesthetized with a mixture of promethazine (2.5 mg/kg) and ketamine hydrochloride (44 mg/kg) administered intramuscularly. Intravenous access was gained via a marginal ear vein and pentobarbital was given intravenously to maintain anesthesia. VX2 tumor tissue was excised from the carrier rabbit and placed in Hanks solution. Abdomens of the recipient rabbits were shaved and prepared with povidone iodine, and then a midline subxyphoid incision was made. The anterior surface of the liver was exposed and the harvested tumor cells (0.1-0.2 cm3) were implanted onto the left lobe of the liver using a 21-gauge angioneedle. This method allowed the growth of a solitary, well-demarcated tumor. The abdomen was closed in two layers. Proper aseptic technique was rigorously observed during each implantation. After surgery, the animals were returned to their cages, kept warm, and monitored in the animal laboratory until they recovered from anesthesia. Buprenorphine (0.01 mg) was administered for analgesia when the animals showed pain or physical distress. The tumors were allowed to grow for another 12 d when they reached a mean diameter size of 1.0-2.0 cm. No animal died during that phase of the protocol.
After 2-wk implantation of VX2 tumors in the rabbit livers, transcather hepatic arterial embolization was performed. A total of 24 animals were treated in such a manner. The animals were brought to the angiography table. Surgical incision was done in order to gain access to the right common femoral artery, into which a 3-F sheath was placed. A specially manufactured 3-F catheter with a tip in the shape of a hockey stick (Terumo, Japan) was manipulated into the celiac axis, after which celiac arteriography was performed to delineate the blood supply to the liver and confirm the location of the tumor. In all cases, the tumor could be readily visualized as a region of hypervascular blush located on the left side, near the gastric fundus. The left hepatic artery, which nearly exclusively provides flow to the tumor, was selectively catheterized off the common hepatic artery. When the catheter was adequately positioned within the left hepatic artery, the mixture of drugs was infused into the artery. Group A received Ad-p53 (1 × 1011 VP/10 mL), group B Ad-p53 (1 × 1011 VP/10 mL) and 0.6-0.8 mL lipiodol, group C 0.5mg/kg mitomycin C and 0.6-0.8 mL lipiodol, group D 10 mL sodium chloride, respectively. Digital spot images obtained after interventional therapy demonstrated intense staining of the tumor consistent with excellent distribution of the mixture in the tumor. The catheter was then removed and the common femoral artery was ligated using resorbable suture material to obtain hemostasis. The procedure was successfully performed in all animals. Proper aseptic techniques were observed throughout the procedure. The animals were monitored after the procedure and given analgesics if they showed any sign of physical distress.
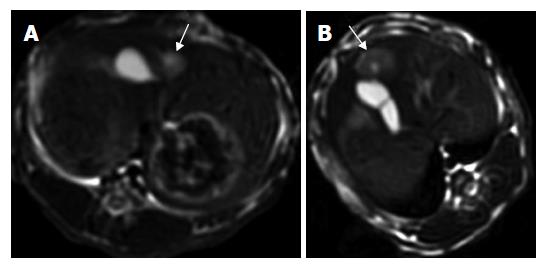
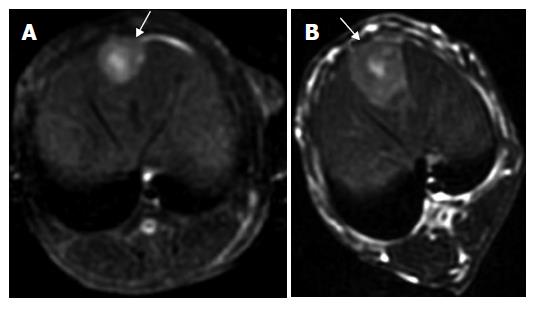
All studies were performed with a 3.0-T GE EXCITEII MR scanner (Milwankee, Wisconsin, USA) using a knee coil before (d 13) and after therapy (d 21). T1-weighted (FSPGR: TR/TE, 120/2.1 ms) and T2-weighted (FSE:TR/TE, 3000/89.2 ms) transverse images with a section thickness of 3 mm, 0.5 mm gap and 128 × 128 matrix were acquired. All images were interpreted regarding tumor size, tumor necrosis and exhepatic metastatic lesions by two radiologists, respectively. No contrast medium was administered. The tumor volume was evaluated and calculated on T2-weighted image according to the following formula[19]: tumor volume (m3) = largest diameter (mm) [smallest diameter (mm)]²/2.
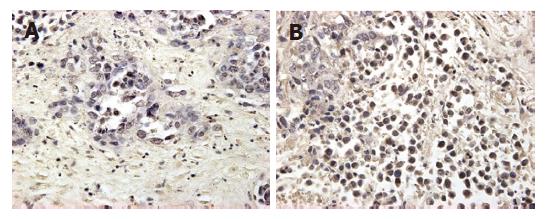
After completion of the MR imaging experiments, the liver was carefully dissected and excised. The liver containing tumor nodules, which were easily identifiable as hard white areas, was prepared for histologic examination. The formalin-fixed, paraffin-embedded specimens were examined immunohistochemically using mutant-type p53 antibody BAO521 at dilution: 1:100 (Boster Co., Wuhan, China). Positive controls were selected cases known to be positive for primary antibody (supplied by Boster Co.). Negative controls were stained with a nonspecific IgG (normal goat IgG) and Tris-buffered saline. Brown-yellow staining in nuclei of cancer cells was found in p53 positive cells. All slides were reviewed with JD morphology image analysis system (JD801, TSJEDA Technology, Inc) and percentage of positive expression was scored by two independent observers in blind. A few cases with discrepant scores were reevaluated to reach a final agreement.
The mean growth ratio of V2/V1 and the p53 protein expression were analyzed by using Dunnett’s test (SPSS, USA) comparing the effect of each therapeutic group. P < 0.05 was considered statistically significant.
The rate of tumor implantation reached 100%. None of the animals died during implantation or interventional therapy. All the 24 VX2 tumors were seen with no enhanced MR imaging (100%) before treatment.
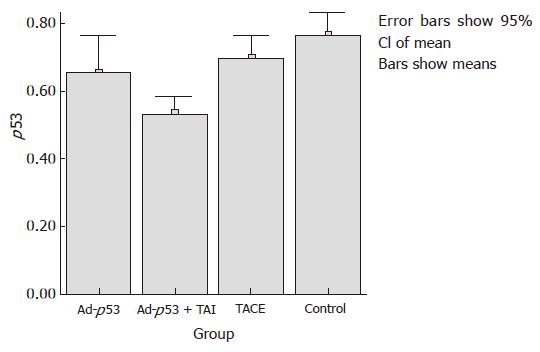
The mean ratio of V2/V1 was 2.35 ± 0.59 in group A, 1.75 ± 0.28 in group B, 2.00 ± 0.44 in group C and 3.14 ± 0.64 in group D. Compared to group D, the other groups all showed a significant decrease in the tumor growth ratio (P < 0.05) using Dunnnett’s t test. A slight difference in the tumor growth ratio was found between groups A and B (P = 0.048). Group A showed a little increase compared to group C (P > 0.05). There was no statistically significant difference between groups B and C (Table 1).
| No. | Group A p53 | Group B p53 + TAE | Group C TACE | Group D | ||||||||
| V1 | V2 | V2/V1 | V1 | V2 | V2/V1 | V1 | V2 | V2/V1 | V1 | V2 | V2/V1 | |
| 1 | 847 | 1296 | 1.53 | 650 | 908 | 1.40 | 1714 | 2267 | 1.32 | 580 | 1912 | 3.30 |
| 2 | 527 | 1008 | 1.91 | 196 | 320 | 1.63 | 879 | 2001 | 2.28 | 908 | 3402 | 3.75 |
| 3 | 405 | 936 | 2.31 | 612 | 1150 | 1.88 | 370 | 886 | 2.39 | 445 | 1089 | 2.45 |
| 4 | 900 | 2268 | 2.52 | 445 | 962 | 2.16 | 1470 | 3402 | 2.31 | 582 | 1538 | 2.64 |
| 5 | 385 | 1250 | 3.25 | 847 | 1296 | 1.53 | 786 | 1328 | 1.69 | 908 | 2468 | 2.72 |
| 6 | 446 | 1152 | 2.58 | 527 | 1008 | 1.91 | 668 | 1524 | 2.28 | 850 | 3402 | 4.00 |
One intrahepatic metastasis lesion in group A and two intrahepatic foci in group D were detected respectively at T2 weighted imaging. Hyper-dense areas on the T2-weighted images, probably due to intratumoral necrosis with bleeding , occurred in three of six rabbits in groups B and C, respectively (Figures 1 and 2).
The mean expression of mutant-type p53 was 0.66 ± 0.11 in group A, 0.53 ± 0.05 in group B, 0.70 ± 0.06 in group C and 0.77 ± 0.07 in group D. The positive cell rate of mutant-type p53 was the lowest in group B, and differed significantly from that in groups A and C (P < 0.05). Compared to the control group, groups A and C showed a decrease in the expression of mutant-type p53, but there was no significant difference between them (Figures 3 and 4).
Rabbit VX2 tumor model has been well established for many years. Because its vascularization is similar to that of human liver tumor, it has been used in clinical research on liver tumor imaging, chemotherapy and tumor etiology[20]. VX2 tumor is hypervascular, and cellular necrosis is more easy to occur at its early stage, which may influence the outcome evaluation of therapy. So this study applied the method of performing MR examination one week after interventional therapy. Because the tumor volume is different before therapy, we took the ratio of V2/V1 as the evaluation index for tumor growth.
The wild-type p53 gene is a tumor suppressor gene involved in the control of cell proliferation. Loss of wild-type p53 function is associated with the uncontrolled growth of many types of human cancer. The reintroduction and expression of wild-type p53 into p53-altered tumor could suppress tumor growth or induce apoptosis both in vitro and in vivo. In the present study, mutant-type p53 protein was detected in all VX2 tumors by immunohistochemical method. Ad-p53 is a recombinant adenoviral vector containing the wild-type human p53 gene. The wild-type p53 protein brings specific anti-tumor cells into effect by inducing apoptosis or necrosis, immune response, or by regulating cell cycle. Recently some studies have used Ad-p53 to suppress tumor growth in liver[17-18]. In our study, Ad-p53 therapy also suppressed the tumor growth in liver. Histological examination revealed the low expression rate of mutant-type p53 protein in group A, as shown in TACE therapy. Wild-type p53 gene can express normal p53 protein. However, normal p53 protein stays for a short time in blood and tissue and degrades quickly, making it hard to be detected. On the contrary, mutant-type p53 protein produced by mutant-type p53 tumor cells is easy to be found in tissue. We took the mutant-type p53 protein to reflect the effective of tumor reduction indirectly. Combined Ad-p53 therapy with trans-arterial embolization could inhibit tumor growth, suggesting that tumor growth is related with the local concentration of Ad-p53. Because of high-velocity blood stream, Ad-p53 gene drug may stay in the tumor for a short time and cannot exert its antiproliferative effects on liver tumor cells. Iodized oil could embolize the local vessels to slow the washout of local drug, and significantly enhance the anticancer effect of Ad-p53 gene drug.
TACE has become one of the most common inter-ventional therapies. It has been shown to result in regres-sion of HCC and reduction of systemic toxicity, thereby improving the therapeutic effects[21]. Some patients with unresectable HCC cannot endure the effect of chemo-therapeutics, and some patients are resistent to the chemo-therapy. However, they may receive the treatment with trans-arterial Ad-p53 and TAE instead of TACE. We should pay attention to the toxicity of Ad-p53.
In summary, combined TAE and Ad-p53 can suppress the growth of VX2 tumors and can be used in the treatment of HCC. However, more accurate and specific evaluation methods are required.
S- Editor Wang J L- Editor Wang XL E- Editor Liu Y
| 1. | Cha C, DeMatteo RP, Blumgart LH. Surgery and ablative therapy for hepatocellular carcinoma. J Clin Gastroenterol. 2002;35:S130-S137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Tang ZY. Treatment of hepatocellular carcinoma. Digestion. 1998;59:556-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Alsowmely AM, Hodgson HJ. Non-surgical treatment of hepatocellular carcinoma. Aliment Pharmacol Ther. 2002;16:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Durand F, Belghiti J. Liver transplantation for hepatocellular carcinoma. Hepatogastroenterology. 2002;49:47-52. [PubMed] |
| 5. | Poon RT, Fan ST, Tsang FH, Wong J. Locoregional therapies for hepatocellular carcinoma: a critical review from the surgeon's perspective. Ann Surg. 2002;235:466-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 308] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 6. | Denys AL, De Baere T, Kuoch V, Dupas B, Chevallier P, Madoff DC, Schnyder P, Doenz F. Radio-frequency tissue ablation of the liver: in vivo and ex vivo experiments with four different systems. Eur Radiol. 2003;13:2346-2352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 94] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Lee JM, Lee YH, Kim YK, Kim SW, Kim SH, Han JK, Choi BI. Combined treatment of radiofrequency ablation and acetic acid injection: an in vivo feasibility study in rabbit liver. Eur Radiol. 2004;14:1303-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Buscarini E, Buscarini L. Radiofrequency thermal ablation with expandable needle of focal liver malignancies: complication report. Eur Radiol. 2004;14:31-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 64] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 9. | Sturm JW, Keese MA, Bönninghoff RG, Wüstner M, Post S. Locally ablative therapies of hepatocellular carcinoma. Onkologie. 2001;24 Suppl 5:35-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Achenbach T, Seifert JK, Pitton MB, Schunk K, Junginger T. Chemoembolization for primary liver cancer. Eur J Surg Oncol. 2002;28:37-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734-1739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2502] [Cited by in RCA: 2611] [Article Influence: 113.5] [Reference Citation Analysis (0)] |
| 12. | Vogl TJ, Trapp M, Schroeder H, Mack M, Schuster A, Schmitt J, Neuhaus P, Felix R. Transarterial chemoembolization for hepatocellular carcinoma: volumetric and morphologic CT criteria for assessment of prognosis and therapeutic success-results from a liver transplantation center. Radiology. 2000;214:349-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 124] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 13. | Lin DY, Lin SM, Liaw YF. Non-surgical treatment of hepatocellular carcinoma. J Gastroenterol Hepatol. 1997;12:S319-S328. [PubMed] |
| 14. | Chen GG, Merchant JL, Lai PB, Ho RL, Hu X, Okada M, Huang SF, Chui AK, Law DJ, Li YG. Mutation of p53 in recurrent hepatocellular carcinoma and its association with the expression of ZBP-89. Am J Pathol. 2003;162:1823-1829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Kouraklis G. Progress in cancer gene therapy. Acta Oncol. 1999;38:675-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Lowe SW, Bodis S, McClatchey A, Remington L, Ruley HE, Fisher DE, Housman DE, Jacks T. p53 status and the efficacy of cancer therapy in vivo. Science. 1994;266:807-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1049] [Cited by in RCA: 1034] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 17. | Guan YS, Liu Y, Sun L, Li X, He Q. Successful management of postoperative recurrence of hepatocellular carcinoma with p53 gene therapy combining transcatheter arterial chemoembolization. World J Gastroenterol. 2005;11:3803-3805. [PubMed] |
| 18. | Sze DY, Freeman SM, Slonim SM, Samuels SL, Andrews JC, Hicks M, Ahrar K, Gupta S, Reid TR. Dr. Gary J. Becker Young Investigator Award: intraarterial adenovirus for metastatic gastrointestinal cancer: activity, radiographic response, and survival. J Vasc Interv Radiol. 2003;14:279-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Carlsson G, Gullberg B, Hafström L. Estimation of liver tumor volume using different formulas - an experimental study in rats. J Cancer Res Clin Oncol. 1983;105:20-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 223] [Cited by in RCA: 225] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 20. | Ramirez LH, Zhao Z, Rougier P, Bognel C, Dzodic R, Vassal G, Ardouin P, Gouyette A, Munck JN. Pharmacokinetics and antitumor effects of mitoxantrone after intratumoral or intraarterial hepatic administration in rabbits. Cancer Chemother Pharmacol. 1996;37:371-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Yoshida T, Sakon M, Umeshita K, Kanai T, Miyamoto A, Takeda T, Gotoh M, Nakamura H, Wakasa K, Monden M. Appraisal of transarterial immunoembolization for hepatocellular carcinoma: a clinicopathologic study. J Clin Gastroenterol. 2001;32:59-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |