Published online Mar 28, 2007. doi: 10.3748/wjg.v13.i12.1867
Revised: December 18, 2006
Accepted: March 10, 2007
Published online: March 28, 2007
AIM: To explore the relationship between metastasis and vagina vasorum in the progress of gastric carcinoma and to find some facts and references for gastric surgeons.
METHODS: One hundred and seven specimens of left or right gastric arteries (55 left and 52 right) were gathered from 59 patients undergoing radical gastrectomy for gastric carcinoma. All the frozen specimens were cut into 3 μm-thick sections and stained with hematoxylin-eosin (HE) and immunohistochemical method separately. Cytokeratin (CK) and mesothelial cells (MC) were stained with immunohistochemical method. Cancer cells inside vagina vasorum were detected and the structure of artery wall was observed under microscope.
RESULTS: Metastatic cancer cells or tubercles were found inside vagina vasorum in some stage III or IV specimens, but not in stageIor II specimens. Tumor cells in vagina vasorum were CK positive in 26 specimens of 14 tumors. Among them, stage III was found in 4 specimens of 2 tumors, and stage IV in 22 specimens of 12 tumors. None of these specimens was positive for MC. The positive rate of CK increased with TNM staging. Compared with the lower part, tumors in the upper and middle parts of stomach were more likely to metastasize into vagina vasorum.
CONCLUSION: Vagina vasorum dissection should be performed during D2 lymphadenectomy for TNM stage III or IV gastric carcinoma.
- Citation: Peng JJ, He YL, Zhan WH, Xiao P, Cai SR, Zhang CH, Wu H. Vagina vasorum dissection during D2 lymphadenectomy for gastric carcinoma. World J Gastroenterol 2007; 13(12): 1867-1869
- URL: https://www.wjgnet.com/1007-9327/full/v13/i12/1867.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i12.1867
Whether vagina vasorum should be dissected is a hot problem. Extended lympathadenetomy (D2) is the standard radical gastrectomy for gastric carcinoma[1,2]. Now more and more gastric surgeons want to reach a consensus on this. However, vagina vasorum is a layer with tough dense connective tissue surrounding vessels. We want to know the function of vagina vasorum around gastric arteries during the progress of gastric carcinoma. We designed this study to elucidate the function of vagina vasorum around gastric arteries during the progress of gastric carcinoma and to find the relationship between vagina vasorum and metastasis of gastric cancer cells.
One hundred and seven specimens of left or right gastric arteries (55 left and 52 right) were collected consecutively from 59 gastric cancer patients. All patients underwent radical gastrectomy for gastric carcinoma in the First Affiliated Hospital of Sun Yat-Sen University. These cases included TNM stagesI-IV gastric cancer. Thirty-three patients were male (55.93%) and twenty-six were formale (45.07%). Their average age was 55.2 ± 13.4 years. Pathologic grading was done after each radical gastrectomy for gastric cancer (Table 1).
| TNM stage | Patients (n) | Gastric arteries | % | |
| GLA | GRA | |||
| I | 6 | 5 | 5 | 10.2 |
| II | 13 | 11 | 12 | 22.0 |
| III | 17 | 16 | 14 | 28.9 |
| IV | 23 | 23 | 21 | 38.9 |
All the 107 frozen specimens were cut into 3 μm–thick sections. The sections were divided into 3 groups (A-C). All the specimens were stained with hematoxylin-eosin (HE) to find metastatic cancer cells inside vagina vasorum of the left and right gastric arteries. Cytokeratin was used in immuno-histochemical method for its sensitivity to epithelial tumor. At the same time, we also used mesothelial cells (MC) to eliminate mesothelial cell cancer. The sections were examined under light microscope.
All data were analyzed with SPSS11.0 for windows. The comparison of two sets of discrete differential counts was done with chi square test. P < 0.05 was considered statistically significant.
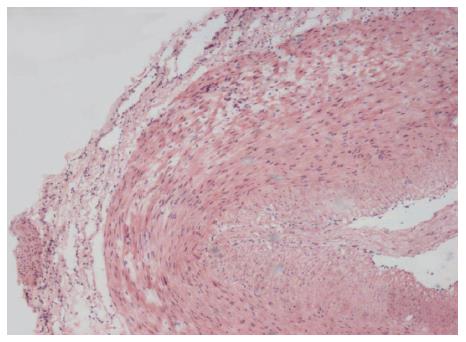
Vagina vasorum structure and red blood cells in vessels could be found in 107 specimens with HE staining. Even metastatic cancer tubercles were found inside vagina vasorum (Figure 1).
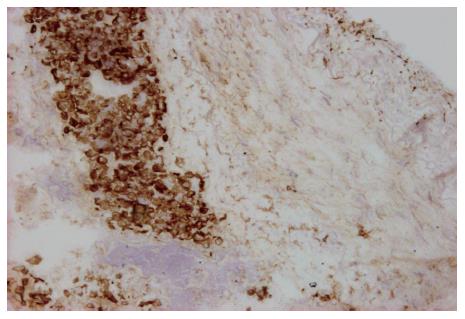
Metastatic cancer cells or tubercles inside vagina vasorum were cytokeratin (CK) positive in 26 specimens of 14 tumors. Among them, stage III was found in 4 specimens of 2 tumors, and stage IV in 22 specimens of 12 tumors (Figure 2). No cancer cell was found in stageIor II specimens of vagina vasorum. All the cancer cells were stained brown with CK. However, no malignant mesothelium was found, as none of them was MC positive.
With the progression of gastric cancer, cancer cells would move into the vagina vasorum around gastric arteries. In this study, we compared CK positive rates in different TNM stages (Table 2). Fifty-nine specimens met the criteria for the TNM staging in CK positive cases. The positive rate of CK was higher in stages III and IV than in stagesIand II (P < 0.05). Moreover, there was a significant difference between stages III and IV (P < 0.05).
| TNM staging | CK | Total | CK positive rate (%) | P | P | |
| Positive | Negative | |||||
| I | 0 | 6 | 6 | 0 | < 0.05 | |
| II | 0 | 13 | 13 | 0 | ||
| III | 2 | 15 | 17 | 11.8 | < 0.05 | |
| IV | 12 | 11 | 23 | 52.2 | ||
| Total | 14 | 45 | 59 | 23.7 | ||
D2 lymphadenectomy is to dissect lymph nodes. Lymph nodes in the left gastric artery, common hepatic artery and celiac artery were dissected during extended lymphadenectomy. The dissected lymph nodes were counted. The criteria for positive and negative lymph nodes were metastatic cancer cells found in any lymph node and no cancer cells found in lymph nodes, respectively. The CK positive rate in positive lymph nodes was higher than that in negative lymph nodes (Table 3).
| Lymph node | CK | Total | CK positive rate (%) | P | |
| Positive | Negative | ||||
| Positive | 12 | 12 | 24 | 50.0 | < 0.01 |
| Negative | 2 | 33 | 35 | 5.7 | |
The stomach is anatomically divided into upper, middle, and lower thirds. Cases were divided into three groups according to the location of the tumors, except for one case of diffuse infiltrative gastric cancer (Table 4). Metastasis of vagina vasorum tumor in the upper and middle thirds was more common than that in the lower third.
| Location | CK | Total | CK positive rate (%) | P | |
| Positive | Negative | ||||
| Upper | 4 | 8 | 12 | 33.3 | |
| Middle | 4 | 9 | 13 | 30.8 | |
| Lower | 5 | 28 | 33 | 15.2 | < 0.01 |
| Total | 13 | 45 | 58 | 22.4 | |
Several factors can produce a good prognosis of gastric cancer. However, the only way to improve survival rate is to remove the carcinoma tissues thoroughly. During radical gastrectomy for gastric carcinoma, en bloc excision of tumor, dissection of lymph nodes and elimination of dissociative cancer cells in abdominal cavity are the three goals[3]. The Japanese Gastric Cancer Association advised that D2 lymphadenectomy is the standard operation for gastric cancer[1,2]. This has been approved by more and more surgeons in the world. The comparison between D2 and less D2 lymphadenectomy has been reported[4-7]. The 5-year overall survival rate for patients treated with D2 lymphadenectomy is higher than that for those not treated with D2 lymphadenectomy[7-9]. It was reported that modified D2 lymphadenectomy sparing the spleen and pancreas could be performed safely and may prolong the survival of patients[10]. However, the postoperative mortality and morbidity are corresponding in two groups[5,11]. Whether the vagina vasorum of gastric artery should be removed during D2 lymphadenectomy remains controvisial. The vagina vasorum is a layer with tough dense connective tissue surrounding vessels containing a large number of capillaries, lymphangion and a few of neurofibrae[12]. It was reported that if the vagina vasorum was reserved, metastatic cancer cells inside vagina vasorum may be residual, although whether gastric cancer cells metastasize through vagina vasorum cannot be confirmed[3,13]. Rovertson et al[14] reported a prospective randomizaed trial comparing R1 with R3 gastrectomy in 1994, showing that skeletonization of the vessels in R3 gastrectomy for gastric cancer could lead to a high morbidity because of intra-abdominal sepsis. Dong et al[15] presumed that pseudoaneurysm of gastroduodenal artery following radical gastrectomy for gastric carcinoma is caused by the weakness in arterial wall due to the skeletonization resulting from lymphadenectomy.
In the earlier stage of gastric cancer, no cancer cells could be found inside vagina vasorum of gastric artery. However, tumor cells appeared in stage III or IV vagina vasorum tumors, and even cluster of cancer cells could be found in vagina vasorum of artery in some more advanced cancer samples, suggesting that tumor cells have the tendency to metastasize into vagina vasorum during the progression of carcinoma. By comparing results in Table 2 and 3, metastasis of cancer cells inside vagina vasorum increased with the development of gastric carcinoma, especially in patients accompanying lymph node metastasis.
Tumors in the upper and middle thirds of stomach had a greater tendency to metastasize into vagina vasorum compared to gastric cancer in the lower third of stomach. Though we cannot explain the differences, the prognosis of tumors in the lower third of stomach is better than that in any other locations of stomach[16-18]. We presume that the prognosis of gastric cancer is associated with the metastasis of gastric cancer cells into the vagina vasorum.
In conclusion, vagina vasorum around gastric arteries should be dissected during D2 lymphadenectomy for gastric cancer. Metastatic gastric cancer cells should be removed thoroughly. Accurate pre-operative and operative pathologic staging is essential.
S- Editor Liu Y L- Editor Wang XL E- Editor Chin GJ
| 1. | Japanese Classification of Gastric Carcinoma -2nd English Edition. Gastric Cancer. 1998;1:10-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2009] [Cited by in RCA: 1942] [Article Influence: 71.9] [Reference Citation Analysis (0)] |
| 2. | Aiko T, Sasako M. The new Japanese Classification of Gastric Carcinoma: Points to be revised. Gastric Cancer. 1998;1:25-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 42] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | Zhan WH. Normalizing the surgical treatment of gastric malignant tumor. Zhonghua Putong Waike Zazhi. 2000;9:289-291. |
| 4. | Edwards P, Blackshaw GR, Lewis WG, Barry JD, Allison MC, Jones DR. Prospective comparison of D1 vs modified D2 gastrectomy for carcinoma. Br J Cancer. 2004;90:1888-1892. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 73] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 5. | Degiuli M, Sasako M, Ponti A, Calvo F. Survival results of a multicentre phase II study to evaluate D2 gastrectomy for gastric cancer. Br J Cancer. 2004;90:1727-1732. [PubMed] |
| 6. | Sano T, Sasako M, Yamamoto S, Nashimoto A, Kurita A, Hiratsuka M, Tsujinaka T, Kinoshita T, Arai K, Yamamura Y. Gastric cancer surgery: morbidity and mortality results from a prospective randomized controlled trial comparing D2 and extended para-aortic lymphadenectomy--Japan Clinical Oncology Group study 9501. J Clin Oncol. 2004;22:2767-2773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 493] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 7. | Volpe CM, Koo J, Miloro SM, Driscoll DL, Nava HR, Douglass HO. The effect of extended lymphadenectomy on survival in patients with gastric adenocarcinoma. J Am Coll Surg. 1995;181:56-64. [PubMed] |
| 8. | Grau JJ, Palmero R, Marmol M, Domingo-Domenech J, Monzo M, Fuster J, Vidal O, Fondevila C, Garcia-Valdecasas JC. Time-related improvement of survival in resectable gastric cancer: the role of Japanese-style gastrectomy with D2 lymphadenectomy and adjuvant chemotherapy. World J Surg Oncol. 2006;4:53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Volpe CM, Driscoll DL, Miloro SM, Douglass HO. Survival benefit of extended D2 resection for proximal gastric cancer. J Surg Oncol. 1997;64:231-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Swan R, Miner TJ. Current role of surgical therapy in gastric cancer. World J Gastroenterol. 2006;12:372-379. [PubMed] |
| 11. | Stevanović D, Radovanović D, Pavlović I, Mitrović N. Effect of systematic lymphadenectomy on postoperative mortality and morbidity in patients with gastric cancer. Med Pregl. 2004;57:381-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | TakaHashi TaHaSHI. The radical correction for colorectal carcinoma. 1st ed. Beijing: People's Med Pub 2003; 145-175. |
| 13. | Collard JM, Malaise J, Mabrut JY, Kestens PJ. Skeletonizing en-bloc gastrectomy for adenocarcinoma in Caucasian patients. Gastric Cancer. 2003;6:210-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (1)] |
| 14. | Robertson CS, Chung SC, Woods SD, Griffin SM, Raimes SA, Lau JT, Li AK. A prospective randomized trial comparing R1 subtotal gastrectomy with R3 total gastrectomy for antral cancer. Ann Surg. 1994;220:176-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 251] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 15. | Kim DY, Joo JK, Ryu SY, Kim YJ, Kim SK, Jung YY. Pseudoaneurysm of gastroduodenal artery following radical gastrectomy for gastric carcinoma patients. World J Gastroenterol. 2003;9:2878-2879. [PubMed] |
| 16. | Moreaux J, Msika S. Carcinoma of the gastric cardia: surgical management and long-term survival. World J Surg. 1988;12:229-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Ohno S, Tomisaki S, Oiwa H, Sakaguchi Y, Ichiyoshi Y, Maehara Y, Sugimachi K. Clinicopathologic characteristics and outcome of adenocarcinoma of the human gastric cardia in comparison with carcinoma of other regions of the stomach. J Am Coll Surg. 1995;180:577-582. [PubMed] |
| 18. | Saito H, Fukumoto Y, Osaki T, Fukuda K, Tatebe S, Tsujitani S, Ikeguchi M. Distinct recurrence pattern and outcome of adenocarcinoma of the gastric cardia in comparison with carcinoma of other regions of the stomach. World J Surg. 2006;30:1864-1869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 76] [Article Influence: 4.0] [Reference Citation Analysis (0)] |