INTRODUCTION
Fulminant hepatic failure (FHF), which remains a disease with high mortality, cannot be reversed by conventional treatments[1-3]. Meanwhile, the complications of liver failure, such as, encephalopathy, cerebral edema and multi-organ failure made it more difficult to cure. Orthotopic liver transplantation has improved the survival rate to 70%~80% of patients with this almost deadly disease. However, many patients died while waiting for the organ because of the lack of donor liver. Therefore, more curiosity has been aroused to deal this with biological treatments, such as extracorporeal bioartificial liver support system (EBLSS)[2-6]. The shortage of human organs leads to the use of non-human donors as the main hepatic cell source. Porcine hepatocytes, including transgenic pig, have become an important cell source widely used in bioartificial liver support systems. Concerns have been raised about the safety of this potential therapy, especially the possibility of cross species transmission of porcine endogenous retroviruses (PERV) since vitro infection of human cell lines has been demonstrated in 1997[4,8-11]. PERVs are integrated into the genome of all pigs. They belong to the gammaretroviruses previously termed type C retroviruses. At least two subtypes of PERV, PERV-A and PERV-B, infect human cells in vitro. Viral particles have been shown to be released by mitogen stimulated porcine peripheral blood mononuclear cells (PBMC), etc[10,12-13]. Clinical trials did not find provirus integration, indicating that no virus infection had taken place. However, further study and more retrospective research should be done in this field.
PERV infection can be measured directly by PCR, RT-PCR[10,13]. Here, we present the methods for the detection of PERV and investigation of patients who had been treated with EBLSS based on porcine cells. All methods were specific and sensitive, and it was revealed that in all patients no PERV infection had occurred.
MATERIALS AND METHODS
Isolation of porcine hepatocytes
Liver cells were isolated by an adaptation of the two-step perfusion method[14,19-20]. Briefly, the animals were anesthetized with barbital (30 mg/kg, b.w, intraperitoneally) and their livers were removed intact. The liver was first perfused in vitro via the portal vein with warmed (37 °C) Ca2+ and Mg2+ free Hanks balanced salt solution at a flow rate of 5-8 mL/min for 10-15 mL/min, and then perfused with 0.05% collagenase (Sigma, Type IV) in the same solution supplemented with 5 mmol/L CaCl2 and 50 mmol/L HEPES. The reperfusion with collagenase soultion lasted 20 min at a rate of 5 mL/min at 37 °C. After 10 min of incubation (37 °C) with gentle shaking, the suspension was filtered and hepatocytes were sedimented at 50×g for 3 min. The viability of the isolated liver cells was determined by the trypan blue exclusion test.
PERV proviral PCR assays
DNA was extracted from porcine liver tissue using the preparation kits (Qiagen). For the detection of provirus, primers specific for the gag gene (forward primer 5’-GCGACCCACGCAGTTGCATA-3’, and reverse primer 5’-CAGTTCCTTGCCCAGTGTCCTT-3’) were used, and a second PCR was carried out to test the products using the primers 5’-TGATCTAGTGAGAGAGGCAGAC-3’ and 5’-CGCACACTGGTCCTTGTCG-3’. For PCR amplification, the standard PCR program of one cycle of 95 °C for 10 min, 35 cycles of 95 °C for 1 min, 55 °C for 1 min, and 72 °C for 1 min and one cycle of 72 °C for 7 min was applied. Liver tissues of human and experimental animals (rabbit and mice) were also gathered to analyze the specificity of PCR.
RT-PCR detection of PERV gag RNA sequences
For the detection of virus released from the cultured cells, viral RNA from supernatants of cultured cells was isolated. Cells were removed from supernate by centrifugation at 200×g for 10 min, thereafter cell debris was removed by centrifugation at 3 500×g for 10 min and an additional centrifugation step at 10 000×g for 30 min. Viral RNA was extracted using the RNA isolation kits (Qiagen)[15-18]. RNA was reverse transcribed using a one-step RT-PCR kit (promega) with no-RT PCR controlled.
Porcine hepatocytes cultured in fiber weaven bioreactor
Primary cells were harvested from 1 wk China Experimental minipigs by a modified two-step collagenase perfusion method[19]. After testing the viability of the cells by trypan blue exclusion, single porcine hepatocyte suspensions were cultivated in the extralumen of the fiber weaven bioreactor (developed by our institute (porous, 0.2 μm)). The bioreactor was filled with porcine hepatocyte about 1.0×108~1.4×109 in co-culture with non-parenchymal cells. DMEM containing EGF was circulated with an artificial capillary cell culture system from Cellmax. Both the biological function and enzyme release of the bioreactor were examined.
Patients
Patients included in the study were FHF, which was defined as occurring within 8 wk of onset of the precipitation illness (in the absence of pre-existing liver disease), and acute-on-chronic hepatic failure listed for liver transplantation with a progressive hepatic encephalopathy.
Extracorporeal liver support with the CellMax bioreactor
FHF patients were treated with hybrid liver support system, including a blood circuit with a continuous plasma separation unit and a second circuit for plasma perfusion of the CellMax bioreactor. Briefly, the bioreactor was filled with porcine hepatocyte about 6.0×108-1.4×109 in co-culture with non-parenchymal cells. Venous blood of FHF patients was elicited via a double-lumen dialysis catheter from the internal jugular vein and blood plasma were separated. Continuous hollow fibre plasma separation was performed at a rate of 100-150 mL/min and then stored in sterile chamber. For anticoagulation a continuous infusion of heparin was performed. After one or two days’ circulatory culture, the bioreactor was then connected and plasma was continuously perfused at a rate of 150-200 mL/min from the chamber. Treated plasma was reunited with the blood cell and returned to the patients. A roller pump (Millipore ultrafiltration device) was used to circulate blood and a heater was used to maintain the temperature of patients and bioreactor at 37 °C-39 °C. After the perfusion, the patients’ plasma samples were detected. Blood samples were obtained after the treatment.
In vitro infection experiments
For infection experiments, cell-free supernatants from cultured porcine hepatocytes were used to infect fetal liver cells in order to mimic the bioartificial liver support system in vitro. That is, cell-free supernatants from primary cultured porcine hepatocytes were collected and cell debris was removed by centrifugation at 10 000× for 30 min, then was used to infect fetal liver cells.
RESULTS
Cell yield and viability
The total isolated hepatocytes and non-parenchymal liver cells by the simplified two step perfusion method were 6.0-14.0×108 cell per liver. The estimated viability judged by the trypan blue test was 92%-96%.
Specificity of the PERV DNA assays
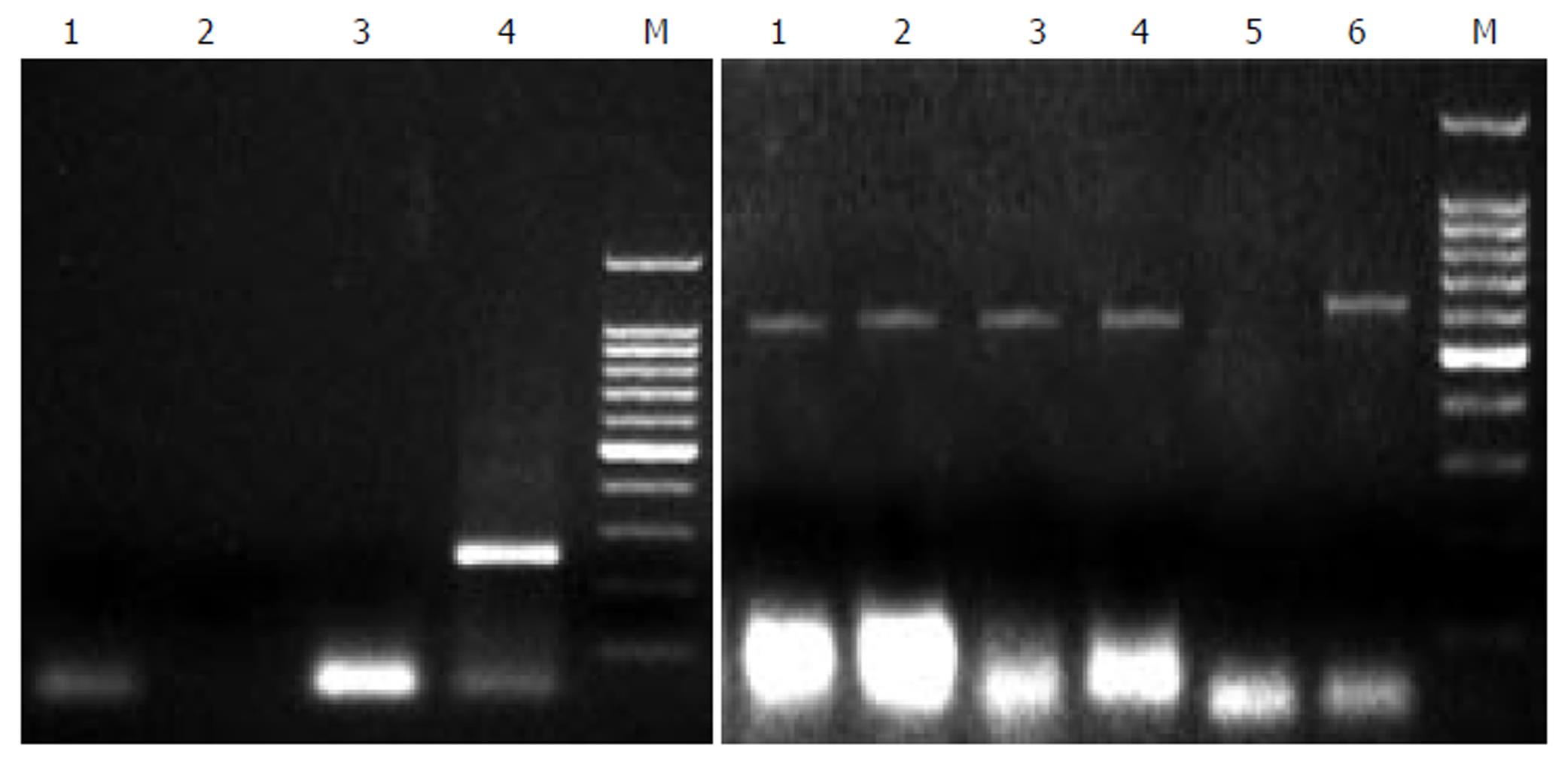
All these PCR assays gave negative results on tissue and serum samples from 2 HBV patients, as well as 2 HCV patients and 1 rabbit, and positive results on tissue of China experimental swine, demonstrating 100% sensitivity, as shown in Figure 1. The detection of product with second PCR gave the same results, showing the usefulness and specificity of the method in observing PERV infection.
Figure 1 PCR analysis of PERV proviral DNA in different liver tissues.
Left: 1: normal person liver tissue, 2: HBV positive liver tissue, 3: HCV positive liver tissue, 4: second PCR of pig liver tissue, 5: Marker. Right: 1, 2, 3, 4, 6: pig liver cells, 5: nagative control.
PERV release of cultured porcine hepatocytes in bioreactor
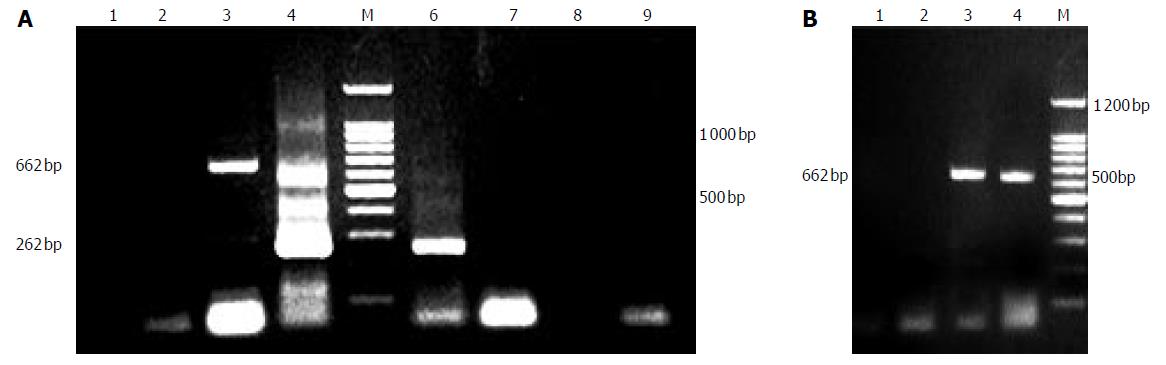
As depicted in Figure 2A, PERV could be released from porcine hepatocytes cultured not only in common flasks but also in fiber weaven bioreactor without the stimulation of mitogen. In the early stage, the quantity of virus so small that it couldnot be detected by PCR assays. From third day on, a large amount of PERVs could be released from the cells till their death. We also found that the semipermeable membrane (0.2 um) of bioreactor couldnot separate the virus, because in the both sides of the lumen we had evidence of the existence of PERV (Figure 2B).
Figure 2 A: PERV RNA detection of supernate.
1:supernate of d 1, 2: supernate of d 3; 3:no RT PCR of d 3; 4:second PCR of d 3; 6: second PCR of d 5; 7: RT-PCR of d 7; 8: RT-PCR of d 9; 9: no RT PCR of d 9; B: PERV permeation of bioreactor. 1: intraluminal supernate of d 3 (no RT PCR), 2: extraluminal supernate of d 3 (no RT PCR), 3: intraluminal supernate of d 3 (RT-PCR); 4: extraluminal supernate of d 3 (RT-PCR).
PERV detection of patients using EBLSS
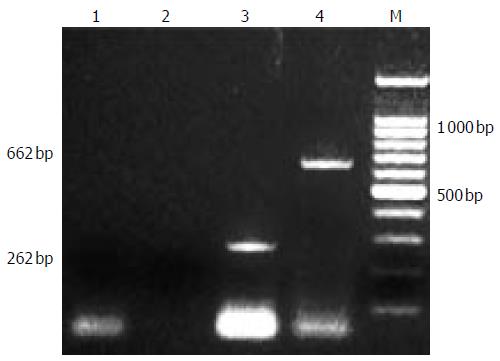
Continuous EBLSS treatment over a period of 4-6 h was safely performed and well tolerated by all the patients. No complications associated with therapy were observed during the treatment and the follow-up period of 1-2 years. After treatment, patients’ blood was obtained for screening with the method mentioned above. No PERV RNA was detected in the serum of any of the 3 patients (Figure 3).
Figure 3 infection of PERV.
1,2 RT-PCR and second PCR of cultured fetal hepatocytes, 3,4: positive control.
PERV infection experiments in vitro
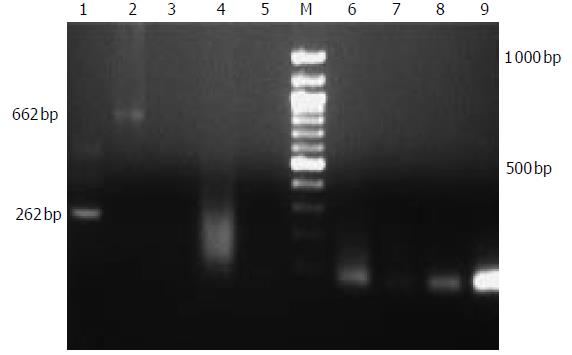
PERV infection experiments with human fetal liver cells were performed. Supernatants from the cultured porcine hepatocytes were positive for PERV detection. Although it has been demonstrated that human kidney cells can be infected, it was also important to evaluate PERV infection of other human cells. In all fetal cells at one week post-infection, no evidence showed that PERV infection occurred with PCR and RT-PCR assays ( Figure 4).
Figure 4 PERV detection of patients treated with EBLSS.
1:second PCR of positive control, 2: positive control, 3:control; 4-9:RT-PCR and second PCR of patients.
DISCUSSION
EBLSS based on porcine hepatocytes catches more interest as an effective temporary treatment to improve the chance of survival and a bridge to liver transplantation[5,6,20-24,38-41]. Because of the shortage of human cells, porcine endogenous retroviruse (PERV), found in 1970’s, was focused for its safety in xenotransplantation and treatments based on porcine tissues or cells as vitro infection of human cell lines HEK 293 was demonstrated in 1997[4,34-37]. Laboratory surveillance of PERV infection in pig xenograft recipients and the treatments is critical for determining the safety of pig xenotransplantation. We used here highly specific PCR based assays for the molecular detection of PERV DNA and RNA sequences. In addition, we included a control PCR reaction without RT to confirm that the positive RT-PCR results were due to PERV RNA. we have developed polymerase chain reaction (PCR) and reverse transcription polymerase chain reaction (RT-PCR) assays to detect proviral PERV gag sequences and PERV RNA sequences in serum samples by using specific primers. All these PCR assays gave positive results on tissue of China experimental swine, and the identification of product with second PCR gave the same results, showing the sensitivity and specificity of this method in observing PERV infection.
Viral particles have been shown to be released by pig PBMC, by cultured aorta endothelial cells stimulated with mitogen[10,12,13,25,26]. In several trials, human diabetic patients who were treated with pig pancreatic islet cells, with pig skin or with extracorporeal perfusion of pig liver did not show provirus integration[27,42], indicating that no virus infection had taken place. In vitro infection experiments revealed that PERVs infect not only human cell lines and primary cells, but also a variety of cells of other species including non-human primates. However, no transmission of PERVs in vivo was observed in non-human primates and several small animals gave high doses of PERV. Additional data showing the same results about the safety of PERV have been obtained from several experiments[16,29]. Nevertheless, considering the results of initial clinical xenotranplantations in humans, which found that persistent microchimerism was observed in 23 patients( totally 160) for up to 8.5 years, and the establishment of PERV infection model[27,28], it was worth paying more attention to its safety in xenotransplantation and EBLSS.
In this study, the retrospective investigation of the three patients with the treatment of EBLSS based on porcine hepatocytes was performed for possible PERV transmission. Our data are in agreement with other studies showing lack of PERV transmission. And in laboratory experiment we also found PERV could not be prevented by the hollow fibre membranes, indicating different opinion from the foregoing research that PERV particles could be prevented by using PES hollow fibre membranes with a molecular weight cut-off of 400 000 kD[31]. In fact, the pore size of bioreactor should be large enough to make material exchange possible, so, any efforts trying to block the virus with semipermeable membrane was probably in vain. The lack of PERV transmission in the patients investigated could be owing to short-term contacting with the EBLSS, lasting for 4-6 h during the treatments. And an effective inactivation of PERV released from pig cells may be another reason. Although cells of human could be infected productively with PERV as reported, no transmission was observed in patients treated with EBLSS in our study.
The latest data about the safety of xenotransplantation using porcine cells, tissues and organs has been obtained from experiments involving transplantation of encapsulated pig islet cells into diabetic rats and assessment of PERV infection in patients treated with a bioreactor based on porcine liver cells[29-33], demonstrating no PERV transmission occured. Although no data indicated that PERV infection had occurred in any of the patients treated with the BALSS containing porcine hepatocytes, longer and larger patient follow-up is required to supervise porcine retroviruses and EBLSS.