Published online Feb 21, 2006. doi: 10.3748/wjg.v12.i7.1140
Revised: July 2, 2005
Accepted: July 12, 2005
Published online: February 21, 2006
AIM: To evaluate the effect of antisense vascular endothelial growth factor (VEGF) RNA (PCMV-FGEV) transfection on the profile of hepatocellular carcinoma (HCC) SMMC-7721 cells in vitro and in vivo.
METHODS: SMMC-7721 cells were transfected with PCMV-FGEV antisense, PCMV-VEGF sense and empty vector plasmid encapsulated by lipofectamine as antisense group, sense group and control group respectively. The positive cell clones were selected with G418. The stable transfection and expression of VEGF in the cells were determined by RT-PCR and immunohistochemistry. Cell proliferation was observed by MTT assay. FACS analysis was used to determine the effect of PCMV-FGEV transfection on cell apoptosis. The growth of transfected cells in vivo was also observed in nude mice.
RESULTS: VEGF expression was reduced in SMMC-7721 transfected with PCMV-FGEV, which was confirmed by RT-PCR and immunohistochemistry. No effect of PCMV-FGEV transfection was found on cell proliferation and cell apoptosis of SMMC-7721 in vitro. The growth of cells transfected with PCMV-FGEV was slow in nude mice and accompanied with obvious apoptosis. The latent time of tumors in the antisense group was 25.0 ± 1.8 d, which was longer than that in sense and control groups (F = 19.455, P < 0.01). The average tumor weight in antisense group (0.96 g ± 0.28 g) was the smallest among the three groups (F = 21.501, P < 0.01).
CONCLUSION: The expression of VEGF can be inhibited by antisense PCMV-FGEV. Antisense PCMV-FGEV has no effect on cell proliferation and apoptosis of SMMC-7721 in vitro but can inhibit tumor growth and induce cell apoptosis in vivo.
-
Citation: Hao JH, Yu M, Li HK, Shi YR, Li Q, Hao XS. Inhibitory effect of antisense vascular endothelial growth factor RNA on the profile of hepatocellular carcinoma cell line
in vitro andin vivo . World J Gastroenterol 2006; 12(7): 1140-1143 - URL: https://www.wjgnet.com/1007-9327/full/v12/i7/1140.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i7.1140
Angiogenesis plays an essential role in the proliferation and metastasis of tumor cells by supplying them with nutrition and oxygen and disposing waste products. Vascular endothelial growth factor (VEGF) is an important element in angiogenesis and permeability in normal and pathological tissue [1-6]. Hepatocellular carcinoma (HCC) with an extremely poor prognosis is known to have abundant blood supply. VEGF has been reported to play an important role in the angiogenesis of HCC[7-11]. Due to the high expression level of VEGF mRNA in HCC, antisense RNA is used to elucidate the possible therapeutic effects on HCC. Using this experimental approach, we explored the effects of antisense PCMV-FGEV transfection on the profile of HCC SMMC-7721 cells in vitro and in vivo.
TRIzol, RPMI1640 medium, 10% fetal bovine serum (FBS), lipofectamine, G418 were purchased from GIBCO (Carlsbad, CA, USA). VEGF and factor VIII antibody were purchased from Boster (Wuhan, China). PI and MTT were purchased from Sigma (St. Louis, MO, USA). VEGF sense, antisense vector PCMV-VEGF, PCMV-FGEV and empty vector PcDNA3.1 were given as gifts by NIH, USA.
Twelve female athymic BALB/c-nu/nu nude mice at the age of 4 - 6 wk were purchased from Chinese Academy of Sciences and maintained under conditions that met all requirements for use in an approved facility.
Human HCC SMMC-7721 cells were obtained from the Central Laboratory of Tianjin Cancer Hospital and cultured in RPMI1640 medium containing 100 U/mL penicillin G sodium and 100 U/mL streptomycin sulfate, supplemented with 10% fetal calf serum at 37 °C in a 50mL/L CO2 atmosphere.
Cells were transfected with antisense PCMV-FGEV (antisense group), sense PCMV-VEGF (sense group) and empty vector PcDNA3.1 (control group) encapsulated by lipofectamine. Forty-eight hours after transfection, cells were diluted and plated into tissue culture dishes for 4 wk in complete growth medium containing 400 μg/mL G418. Colonies resistant to G418 were isolated.
Immunohistochemiscal analysis was performed on formalin-fixed, paraffin-embedded sections using a three-step indirect method for VEGF and factor VIII expression.
Guanidinium isothiocyanate-phenol single-step method was used to extract total RNA from cells using TRIzol following instructions of the kit. The integrity, purity and concentration of the extracted mRNA were detected with ultraviolet spectral photometer and agarose gel electrophoresis. The RNA extract dissolved in DEPC was stored at -70 °C. According to the sequences of human mRNA of VEGF, the primers were designed, synthesized and supplied by Boya Biotech, Shanghai, China (VEGF positive primer: 5’ -AATGCTTTCTCCGCTCTG-3’, negative primer:5’-TTGCTGCTCTACCTCCAC-3’ ; β-actin positive primer: 5’-TTGCGCTCAGGAGGAGCAAT-3’, negative primer: 5’-TTCCAGCCTTCCTTCCTGG-3’). First-strand cDNA was synthesized from 2μg RNA dissolved in water administered with DEPC, in which 0.2 μL oligo dT was added at 70°C for 5 min and placed on ice for 1 min, followed by 1μL M-MLV reverse transcriptase (200IU/μL), 0.5μL RNasin (40 IU/μL), 8μL 5×RT buffer, and 3μL dNTPs (10 mmol/L) at 42 °C for 60 min. The reverse transcriptase was inactivated at 95 °C for 5 min, then 5μL cDNA, 5μL 10×buffer, 3μL 2mmol/L dNTPs, 1μL 5 IU/L LA Taq enzyme and water were added to a volume of 40μL. The final concentrations of positive and negative primers were both 1uM/L. VEGF was amplified for 35 cycles at 94 °C for 1 min, at 55 °C for 1 min, at 72 °C for 2 min, and a final extension at 72 °C for 7 min. The area of electrophoresis bands (AREA), the absorption of mean optical density (A) and product of AREA and A were quantitatively analyzed after the PCR product was detected with auto image manipulating system following agarose gel (2%) electrophoresis and stained with EB. The samples were controlled with blank and β-actin.
For analysis of the transfected SMMC-7721 cell proliferation, SMMC-7721 cells were seeded in a 96-well plate (6000 cells/ well), untransfected SMMC-7721 cells were used as negative control. After further incubated for 24, 48, 72, 96, 120, 144 h, cell proliferation activity was determined by MTT assay. The absorbance of each well was measured at 580nm in a microtiter reader. The survival rate of tumor cells was calculated according to the formula: survival rate (%) =(A/ B)×100, where A is the absorbance of treated cells, and B is the absorbance of negative control cells.
To investigate the influence of transfection on apoptosis, cells grown as a monolayer were incubated for 24 h, trypsinized, washed with PBS and fixed with 70% ethanol overnight at 4 °C. Then cells were intensively washed three times with PBS and incubated with 1% RNase for 30 min at 37 °C. Cells were measured with a FACScan flow cytometer (BD Biosciences) equipped with a 488 nm argon-ion laser and a Macintosh Power PC (G4). In general, 25 000 events were acquired using CellQuest Pro 4.0.1. Apoptotic cells were then calculated in percent using ModFIT Vers. 3.0 (BD Biosciences).
Cells were resuspended at the density of 5 × 107 cells in 400 μL of RPMI1640 and injected subcutaneously into the flank region of athymic nude mice. Twelve mice were distributed to antisense group, sense group or negative control group, 4 per group at random. All animals were observed for up to 10 wk following the injection, and then the tumor was excised.
For electron microscopy, small blocks of tumor tissue were fixed in 1% glutaraldehyde and 4% paraformaldehyde, postfixed in 1% osmium tetroxide and embedded in Epon 812, double-stained with uranyl acetate and lead citrate, and observed under a transmission electron microscope.
One-way analysis of variance (ANOVA) was used to compare the results between the different groups. All data were processed with SPSS10.0 statistical software. P < 0.05 was considered statistically significant.
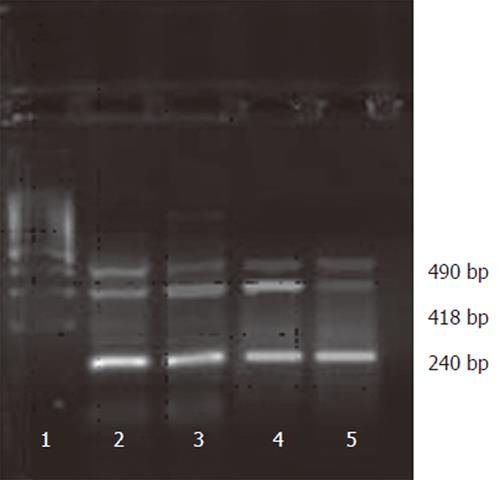
VEGF protein expression was high in sense group, moderate in control and 7721 groups and weak in antisense group (F =16.786, P < 0.01). The same results were also confirmed by VEGF mRNA expression in RT-PCR analysis (Figure 1). The mRNA expression of VEGF was seen in all groups. However, compared to the control and sense groups, the expression was decreased in the antisense group (F = 19.693, P < 0.01), suggesting that SMMC-7721 cells transfected with PCMV-FGEV could effectively inhibit VEGF expression at protein and RNA level.
We used MTT assay to assess the proliferation of transfected SMMC-7721 cells in vitro. The difference in survival rates of the cells in sense group, antisense group, and control group was not significant (F = 0.869, P > 0.05). Further we used flow cytometry to compare the extent of cell apoptosis among the different groups and did not find the characteristic changes of cell apoptosis in each group.
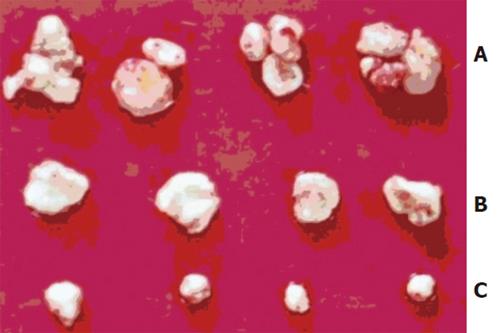
The time of tumorigenesis was 25.0 ± 1.8 d in antisense group, 15.7 ± 2.5 d in sense group and 18.5±2.1 d in control group (F = 19.445, P < 0.01). The weight of tumor was 0.96 ± 0.28 g in antisense group, which was obviously lighter than that in sense group (2.18 ± 0.36g) and control group (1.88 ± 0.47g) (F = 21.505, P < 0.01)(Figure 2).
VEGF immunohistochemical staining showed faint signals in antisense group. The number of positive cells and intensity of staining were significantly lower than those in sense or control group (F = 21.365, P < 0.01). Factor VIII staining showed that the number of tumor vessels in antisene group was significantly less than that in sense or control group (F = 9.985, P < 0.01).
Electron micrographs of the tumor cells in antisense group showed characteristic chromatin condensation forming a crescentic-like cap, which was characteristic appearance of apoptosis. Comparatively, such chromatin change of the cells was not detectable in control or sense group (F = 24.548, P < 0.01).
Hepatocellular carcinoma (HCC) is one of the most common malignant neoplasms. All the treatment strategies used today have no good curative effect [12]. Gene therapy might be a promising way. Gene transfer is a key technique in gene therapy. Antisense gene technologies have been proven to be powerful tools for selective regulation of gene expression in experimental settings and are under evaluation for their therapeutic potential in clinic [13-16]. Antisense agents down-regulate the expression of specific target genes at mRNA level by pairing with their complementary RNA and preventing their translation into proteins. Theoretically, antisense molecules could be used to cure a variety of diseases, especially some cancers[17-20].
VEGF plays an important role in the angiogenesis of HCC. VEGF, known as a vascular permeability factor, has two major biological functions: growth stimulatory activity for a variety of vascular endothelial cells and increasing microvascular permeability [21-23]. Use of antisense VEGF RNA for inhibiting vessel formation in HCC might be a rational approach. Our study showed that antisense VEGF RNA could effectively inhibit the expression of VEGF in HCC cell line SMMC-7721, but could not significantly inhibit growth of the cells in vitro, which is in accordance with the report of Gu et al[24]. The reasons may be as follows: VEGF is specific to endothelial cells and there is no vessel in the HCC cells. Although antisense VEGF RNA can inhibit the expression of VEGF, it can not effectively inhibit tumor cells without vessels. Furthermore If we use the vascular endothelial cells as the model to study the inhibitory effect of antisense VEGF RNA on them, the results may be that antisense RNA could inhibit not only the expression of VEGF but also the growth of vascular endothelial cells.
This study also demonstrated that antisense VEGF RNA could inhibit the growth of tumor in vivo. The secretion of VEGF and the vessels of tumor in antisense group were significantly decreased, leading to inhibition of tumor growth and metastasis. Becker et al[25] reported that transfecting the gene of soluble vascular endothelial growth factor receptor flk-1 to the prostate cancer nude model can inhibit tumor growth and metastasis. Zhang et al [26] found that angiogenic inhibition mediated by DNase targeting at vascular endothelial growth factor receptor -2 could inhibit the formation of vessels and the growth of tumor.
In conclusion, antisense VEGF RNA has inhibitory effect on the growth of HCC cells in vitro and in vivo and can be used in the treatment of HCC.
S- Editor Wang J L- Editor Wang XL E- Editor Wu M
| 1. | Ferrara N, Keyt B. Vascular endothelial growth factor: basic biology and clinical implications. EXS. 1997;79:209-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 2. | Blood CH, Zetter BR. Tumor interactions with the vasculature: angiogenesis and tumor metastasis. Biochim Biophys Acta. 1990;1032:89-118. [PubMed] |
| 3. | Klagsbrun M, D'Amore PA. Vascular endothelial growth factor and its receptors. Cytokine Growth Factor Rev. 1996;7:259-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 299] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 4. | Hemmerlein B, Kugler A, Ozisik R, Ringert RH, Radzun HJ, Thelen P. Vascular endothelial growth factor expression, angiogenesis, and necrosis in renal cell carcinomas. Virchows Arch. 2001;439:645-652. [PubMed] |
| 5. | Li QM, Kan FJ, Min CY. Effect of Weikangning on gastric cancer cell growth and expression of vascular endothelial growth factor and its receptors KDR and Flt-1. World J Gastroenterol. 2005;11:938-942. [PubMed] |
| 6. | Keyes KA, Mann L, Cox K, Treadway P, Iversen P, Chen YF, Teicher BA. Circulating angiogenic growth factor levels in mice bearing human tumors using Luminex Multiplex technology. Cancer Chemother Pharmacol. 2003;51:321-327. [PubMed] |
| 7. | Qin LX, Tang ZY. Recent progress in predictive biomarkers for metastatic recurrence of human hepatocellular carcinoma: a review of the literature. J Cancer Res Clin Oncol. 2004;130:497-513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 147] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 8. | Jia WD, Xu GL, Xu RN, Sun HC, Wang L, Yu JH, Wang J, Li JS, Zhai ZM, Xue Q. Octreotide acts as an antitumor angiogenesis compound and suppresses tumor growth in nude mice bearing human hepatocellular carcinoma xenografts. J Cancer Res Clin Oncol. 2003;129:327-334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Mise M, Arii S, Higashituji H, Furutani M, Niwano M, Harada T, Ishigami S, Toda Y, Nakayama H, Fukumoto M. Clinical significance of vascular endothelial growth factor and basic fibroblast growth factor gene expression in liver tumor. Hepatology. 1996;23:455-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 214] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 10. | Jinno K, Tanimizu M, Hyodo I, Nishikawa Y, Hosokawa Y, Doi T, Endo H, Yamashita T, Okada Y. Circulating vascular endothelial growth factor (VEGF) is a possible tumor marker for metastasis in human hepatocellular carcinoma. J Gastroenterol. 1998;33:376-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 87] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 11. | Tang ZY, Ye SL, Liu YK, Qin LX, Sun HC, Ye QH, Wang L, Zhou J, Qiu SJ, Li Y. A decade's studies on metastasis of hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004;130:187-196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 352] [Cited by in RCA: 362] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 12. | Tang ZY. Hepatocellular carcinoma--cause, treatment and metastasis. World J Gastroenterol. 2001;7:445-454. [PubMed] |
| 13. | Gewirtz AM, Sokol DL, Ratajczak MZ. Nucleic acid therapeutics: state of the art and future prospects. Blood. 1998;92:712-736. [PubMed] |
| 14. | Crooke ST. Molecular mechanisms of action of antisense drugs. Biochim Biophys Acta. 1999;1489:31-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 248] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 15. | Resnicoff M, Li W, Basak S, Herlyn D, Baserga R, Rubin R. Inhibition of rat C6 glioblastoma tumor growth by expression of insulin-like growth factor I receptor antisense mRNA. Cancer Immunol Immunother. 1996;42:64-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Weiss B, Davidkova G, Zhou LW. Antisense RNA gene therapy for studying and modulating biological processes. Cell Mol Life Sci. 1999;55:334-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 67] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 17. | Adjei AA, Dy GK, Erlichman C, Reid JM, Sloan JA, Pitot HC, Alberts SR, Goldberg RM, Hanson LJ, Atherton PJ. A phase I trial of ISIS 2503, an antisense inhibitor of H-ras, in combination with gemcitabine in patients with advanced cancer. Clin Cancer Res. 2003;9:115-123. [PubMed] |
| 18. | Morris MJ, Tong WP, Cordon-Cardo C, Drobnjak M, Kelly WK, Slovin SF, Terry KL, Siedlecki K, Swanson P, Rafi M. Phase I trial of BCL-2 antisense oligonucleotide (G3139) administered by continuous intravenous infusion in patients with advanced cancer. Clin Cancer Res. 2002;8:679-683. [PubMed] |
| 19. | Rudin CM, Otterson GA, Mauer AM, Villalona-Calero MA, Tomek R, Prange B, George CM, Szeto L, Vokes EE. A pilot trial of G3139, a bcl-2 antisense oligonucleotide, and paclitaxel in patients with chemorefractory small-cell lung cancer. Ann Oncol. 2002;13:539-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 111] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 20. | Tolcher AW, Reyno L, Venner PM, Ernst SD, Moore M, Geary RS, Chi K, Hall S, Walsh W, Dorr A. A randomized phase II and pharmacokinetic study of the antisense oligonucleotides ISIS 3521 and ISIS 5132 in patients with hormone-refractory prostate cancer. Clin Cancer Res. 2002;8:2530-2535. [PubMed] |
| 21. | Sugimachi K, Tanaka S, Taguchi K, Aishima S, Shimada M, Tsuneyoshi M. Angiopoietin switching regulates angiogenesis and progression of human hepatocellular carcinoma. J Clin Pathol. 2003;56:854-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 77] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Ramsey DE, Kernagis LY, Soulen MC, Geschwind JF. Chemoembolization of hepatocellular carcinoma. J Vasc Interv Radiol. 2002;13:S211-S221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 163] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 23. | Chow NH, Hsu PI, Lin XZ, Yang HB, Chan SH, Cheng KS, Huang SM, Su IJ. Expression of vascular endothelial growth factor in normal liver and hepatocellular carcinoma: an immunohistochemical study. Hum Pathol. 1997;28:698-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 76] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 24. | Gu ZP, Wang YJ, Li JG, Zhou YA. VEGF165 antisense RNA suppresses oncogenic properties of human esophageal squamous cell carcinoma. World J Gastroenterol. 2002;8:44-48. [PubMed] |
| 25. | Becker CM, Farnebo FA, Iordanescu I, Behonick DJ, Shih MC, Dunning P, Christofferson R, Mulligan RC, Taylor GA, Kuo CJ. Gene therapy of prostate cancer with the soluble vascular endothelial growth factor receptor Flk1. Cancer Biol Ther. 2002;1:548-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Zhang L, Gasper WJ, Stass SA, Ioffe OB, Davis MA, Mixson AJ. Angiogenic inhibition mediated by a DNAzyme that targets vascular endothelial growth factor receptor 2. Cancer Res. 2002;62:5463-5469. [PubMed] |