Published online Feb 14, 2006. doi: 10.3748/wjg.v12.i6.940
Revised: December 1, 2004
Accepted: December 20, 2004
Published online: February 14, 2006
AIM: To evaluate whether treatment with the Prometheus® system significantly affects cytokines, coagulation factors and other plasma proteins.
METHODS: We studied nine patients with acute-on-chronic liver failure and accompanying renal failure. Prometheus® therapy was performed on 2 consecutive days for up to 6 h in all patients. Several biochemical parameters and blood counts were assessed at regular time points during Prometheus® treatment.
RESULTS: We observed a significant decrease of both protein-bound (e.g. bile acids) and water-soluble (e.g. ammonia) substances after Prometheus® therapy. Even though leukocytes increased during treatment (P < 0.01), we found no significant changes of C-reactive protein, interleukin-6, and tumor necrosis factor-α plasma levels (all P > 0.5). Further, antithrombin 3, factor II and factor V plasma levels did not decrease during Prometheus® therapy (all P > 0.5), and the INR remained unchanged (P = 0.4). Plasma levels of total protein, albumin, and fibrinogen were also not altered during Prometheus® treatment (all P > 0.5). Finally, platelet count did not change significantly during therapy (P = 0.6).
CONCLUSION: Despite significant removal of protein-bound and water-soluble substances, Prometheus® therapy did not affect the level of cytokines, coagulation factors or other plasma proteins. Thus, the filters and adsorbers used in the system are highly effective and specific for water-soluble substances and toxins bound to the albumin fraction.
- Citation: Rifai K, Ernst T, Kretschmer U, Haller H, Manns MP, Fliser D. Removal selectivity of Prometheus: A new extracorporeal liver support device. World J Gastroenterol 2006; 12(6): 940-944
- URL: https://www.wjgnet.com/1007-9327/full/v12/i6/940.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i6.940
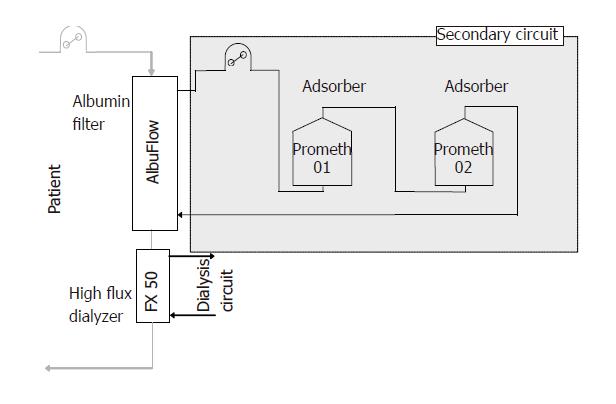
Extracorporeal liver support devices have recently gained much interest. Prometheus® is a newly designed system for extracorporeal treatment of liver failure. It is based upon a recently developed method of albumin dialysis, i.e. fractionated plasma separation and adsorption[1]. First, an albumin-permeable membrane (AlbuFlow, cut-off 250 ku) separates the albumin fraction together with all albumin-bound substances in a secondary circuit. There, direct purification of the plasma is performed by one or two adsorbers (Prometh01 and Prometh02) in order to remove albumin-bound toxins. In parallel, a conventional high-flux dialysis is performed to remove the water-soluble substances.
Results from past studies have documented that extracorporeal liver support devices can remove cytokines and other important plasma proteins[2,3]. Theoretically, removal of these substances may influence spontaneous liver regeneration, similarly as has been proposed for interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α)[4]. Furthermore, development of thrombopenia with an increased risk of bleeding was documented in several patients undergoing albumin dialysis using the MARS system[5]. Until now, there are not sufficient controlled studies available that have evaluated whether treatment with the Prometheus® device may result in an unspecific removal of cytokines or leads to a decrease of coagulation factors or platelets. In addition, albumin or other plasma proteins may be lost during Prometheus® as a result of removal or after clotting in the secondary circuit. Therefore, we performed a prospective clinical study in patients with liver failure and assessed the course of different cytokines, coagulation factors, protein fractions, and thrombocytes during treatment with Prometheus®.
The study was approved by the Ethics Committee of the Hannover Medical School. The Declaration of Helsinki and the rules from Good Clinical Practice were followed. Written informed consent was obtained from all patients or next of kin. Nine patients (four males) with liver failure (five with end-stage cirrhosis, two with acute alcoholic hepatitis, two with liver failure late after liver transplantation) and concomitant renal failure were included in the trial. Their mean age was 52 ± 8 years, and the average APACHE II score was 16.8 ± 3.2. Seven patients (78%) had the highest stage of cirrhosis (C Child-Pugh score) with an average of 11.3 ± 1.7 points. Six patients (67%) were treated in the intensive care unit. All patients were already undergoing hemodialysis and had a functionable double-lumen catheter in place.
Blood coming from the patient by a double-lumen dialysis catheter is pumped into the extracorporeal circuit. There, it passes through an albumin-permeable filter, which is made of polysulfone (AlbuFlow®). The filter has a sieving coefficient that is specially designed. It allows large molecules up to the size of albumin (68 ku) to pass into the secondary circuit. The cut-off is at about
250 ku. Larger molecules cannot pass through the filter. The secondary circuit is filled with isotonic sodium chloride solution. It is driven by a separate plasma pump. By recirculation of the albumin-rich plasma, the filtration and adsorption capacities are enhanced. One or two adsorbers are used to directly purify the filtered albumin-rich plasma: a neutral resin adsorber (Prometh01®) for direct adsorption of the albumin-bound toxins and an anion exchanger (Prometh02®) with chloride used as counterion. The content of the adsorber consists of spherical adsorber particles with 300-1 000 µm in diameter. The adsorbers consist of styrene-divinylbenzene copolymer. Their cylindrical housing has a central inlet and outlet closed at both ends by sieves with a mesh size of 100 µm. The sieves circumvent a passage of the particles through the inlets. Even in case of leakage, they are held back in the secondary circuit by the albumin filter membrane. After the purification of the fractionated plasma in the secondary circuit, the purified plasma is returned to the primary circuit, i.e. into the blood side of the albumin filter. Here the blood is guided through a high-flux hemodialyzer (FX 50®). There, water-soluble substances are removed by diffusion.
A modified hemodialysis unit (4008 H) is used to integrate the two parts of the Prometheus® system (Fresenius Medical Care AG, Bad Homburg, Germany): a “standard” extracorporeal blood circuit connected to the double-lumen dialysis catheter and a secondary circuit containing the separated plasma (Figure 1). Both circuits are separately monitored by different hardware and software allowing the user to perform hemodialysis with simultaneous albumin detoxification or conventional hemodialysis alone.
Instead of their regular hemodialysis sessions, all patients underwent two Prometheus® treatments for at least 4 h on two consecutive days. In 5/9 patients (56%), the device was equipped with two adsorbers (Prometh01® and Prometh02®), while the other four patients (44%) were treated only with Prometh01®. The average treatment time was 5.1 ± 1.1 h, and mean blood flow rate was 193 ± 10 mL/min. The total processed volume within each treatment was 58 ± 14 L. Anticoagulation during therapy consisted of unfractionated heparin administered as a bolus. Because of the increased risk of bleeding in liver patients, heparin administration was restricted to low doses. The activated coagulation time was used to control anticoagulation. It was targeted between 120 and 150. Blood samples for measurement of cytokines (TNF-α and IL-6), platelets, coagulation factors (antithrombin 3, factors II and V) and plasma proteins (total protein, albumin, and fibrinogen) were taken before the start of Prometheus therapy, after 30 min, 2, and 5 h of treatment as well as 24 h after the end of the treatment.
Plasma concentration of C-reactive protein was measured with a clinically validated nephelometric assay (Dade Behring, Germany); the normal range is below 5 mg/L. Plasma concentrations of inflammatory cytokines, e.g. IL-6 and TNF-α, were measured with an ELISA (R&D Systems, USA). The intra-assay coefficients of variation for these tests ranged between 4.9% and 6.8%, respectively. The other biochemical parameters were measured by certified routine analytical methods.
The SPSS program was used for statistical analysis (version 12.0, SPSS, Chicago, USA). All data are presented as mean ± SD. Pre- and post-treatment values were compared using two-tailed t-test for paired data. Correlations were assessed with Pearson’s correlation analysis. A P < 0.05 was considered as statistically significant.
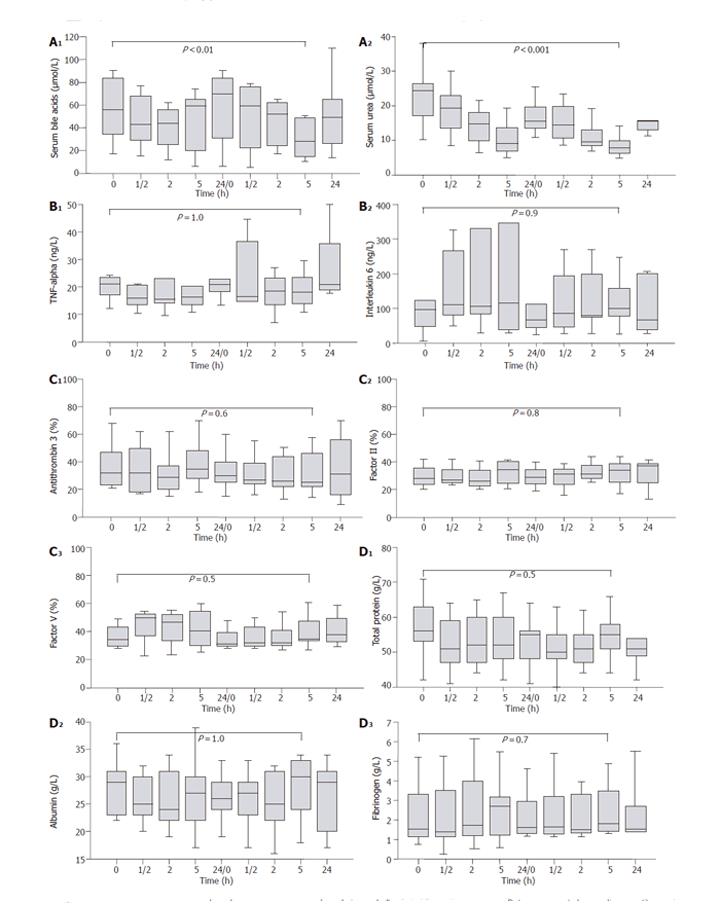
A significant decrease of both protein-bound substances (e.g., bile acids; P < 0.01) and water-soluble substances (e.g., urea; P < 0.001) was observed under Prometheus® therapy (Figure 2A). Even though leukocytes increased from 15.1 × 103 to 22.7 × 103/µL (P < 0.01), there were no significant changes of plasma concentrations of C-reactive protein (P = 0.7), TNF-α (P = 1.0; Figure 2B) or IL-6 (P = 0.9; Figure 2B). TNF-α and IL-6 were correlated with each other (P < 0.03). Furthermore, they were positively associated with body temperature (TNF-α: P < 0.03; IL-6: P < 0.001), but not with C-reactive protein (both P > 0.2). All these parameters were already increased in our patients before Prometheus® therapy.
Platelet count and coagulation factors such as antithrombin 3, factors II and V were substantially decreased in our patients before the start of Prometheus® therapy. However, there was no decrease of platelets during treatment (P = 0.6). Furthermore, the coagulation factors and INR did not significantly change under Prometheus® treatment (all P > 0.4; Figure 2C). All coagulation factors were highly associated with each other (all P < 0.001) and with C-reactive protein (all P < 0.01). A strong positive association was found between factors II and factor V on one hand and leukocyte count on the other hand (both P < 0.001).
Plasma levels of total protein, albumin, and fibrinogen were reduced in liver patients before the start of Prometheus® therapy. Even though we had two episodes of clotting of the secondary, i.e. albumin-filled circuit occurred, we observed no significant alterations of total protein, albumin, and fibrinogen during treatment (all P > 0.5; Figure 2D).
In one patient with hepatitis C cirrhosis and hepatorenal syndrome, we found a marked increase of TNF-α (from 17 to 12 420 ng/L) and IL-6 (from 49 to 15 732 ng/L) already after the 2nd h of treatment on the 1st day of Prometheus® therapy. In parallel, leukocytes decreased from 8.1 × 103 to 1.6 × 103/µL. No specific clinical problems were recorded at that time, so that the Prometheus® treatment was completed. When treatment was stopped, mean arterial pressure was 52 mmHg, whereas body temperature was 38.9 °C and leukocytes 4.4 × 103/µL. At this time point, we later measured already decreasing TNF-α plasma levels
(1 012 ng/L), whereas IL-6 was still increasing (76 250 ng/L). This episode turned out to be a catheter-related bacteremia, and the catheter was removed immediately. After the catheter had been changed, no clinical problems were observed during Prometheus® therapy on the next day. Plasma levels of TNF-α after the second treatment decreased to 49 ng/L and that of IL-6 to 108 ng/L.
Removal selectivity of extracorporeal blood purification devices is a key to assess their specificity and efficacy. Therefore, it is crucial to evaluate whether particular substances are removed or not. With Prometheus® both protein-bound and water-soluble substances that accumulate in liver failure are removed in a single step, i.e. during a single passage of the blood through the extracorporeal circuit. The system is based upon the FSPA method, and in contrast to the MARS system, Prometheus® allows direct purification of the albumin fraction in the secondary circuit[1]. Furthermore, a high-flux dialyzer is incorporated in the Prometheus® system as well.
We were able to show that Prometheus® significantly removed protein-bound (e.g., bile acids) and water-soluble substances (e.g., urea), but we did not observe (unspecific) removal of cytokines such as TNF-α and IL-6. In contrast, it has been shown that both cytokines are removed during MARS treatment[2,3]. This could be of considerable clinical importance, since these pro-inflammatory cytokines are postulated to play a major role also in the process of liver regeneration[4]. Thus, while cytokine removal may have beneficial effects in clinical conditions where activation of pro-inflammatory cytokines is part of the pathophysiological basis of the syndrome, e.g. systemic inflammatory response syndrome (SIRS)[7], it could be potentially even harmful in liver patients by impeding liver regeneration[8]. Since large prospective controlled trials on survival are lacking, it remains to be elucidated whether (unspecific) cytokine removal in liver patients is beneficial or whether these patients benefit more if pro-regenerative cytokines such as TNF-α and IL-6 remain in the body.
Another point of interest in our study was the influence of Prometheus® therapy on coagulation factors and platelets. Usually, patients with liver failure present with moderate to severe coagulopathy. In order to prevent bleeding complications in these patients, the coagulation status should not be further worsened by a removal, activation or consumption of coagulation factors. In none of our patients we observed a decrease of platelets, and coagulation factors such as antithrombin 3, factor II or factor V remained stable throughout the therapy without substitution. This is in contrast to data obtained with the MARS system; it may induce thrombopenia in a significant number of patients with a subsequently elevated risk of bleeding complications[5].
An important point in the evaluation of extracorporeal liver support systems is the possible loss of plasma proteins during treatment. This may result from an unspecific removal, from adsorption on the adsorber, or by a clotting of the secondary circuit that is filled with the patients’ albumin. Even though two episodes of clotting of the secondary circuit occurred in our study, no significant loss of plasma total protein or albumin developed. Furthermore, the selectivity of protein removal was confirmed by measurement of serum fibrinogen concentrations. Fibrinogen molecules are much larger than albumin and should be held in patients’ blood if the sieving coefficient of the membrane used for plasma separation remains unaltered. As expected, fibrinogen levels in our patients remained stable throughout the whole study period.
In conclusion, our data document that the extracorporeal liver support device Prometheus® provides selective removal of protein-bound and water-soluble substances, whereas measured cytokines, coagulation factors or plasma protein fractions as well as platelets remain unaltered. In this aspect the system seems to offer an advantage over the MARS system.
S- Editor Guo SY L- Editor Elsevier HK E- Editor Kong LH
| 1. | Falkenhagen D, Strobl W, Vogt G, Schrefl A, Linsberger I, Gerner FJ, Schoenhofen M. Fractionated plasma separation and adsorption system: a novel system for blood purification to remove albumin bound substances. Artif Organs. 1999;23:81-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 146] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 2. | Stange J, Hassanein TI, Mehta R, Mitzner SR, Bartlett RH. The molecular adsorbents recycling system as a liver support system based on albumin dialysis: a summary of preclinical investigations, prospective, randomized, controlled clinical trial, and clinical experience from 19 centers. Artif Organs. 2002;26:103-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 137] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 3. | Guo LM, Liu JY, Xu DZ, Li BS, Han H, Wang LH, Zhang WY, Lu LH, Guo X, Sun FX. Application of Molecular Adsorbents Recirculating System to remove NO and cytokines in severe liver failure patients with multiple organ dysfunction syndrome. Liver Int. 2003;23 Suppl 3:16-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 85] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 4. | Fausto N. Liver regeneration. J Hepatol. 2000;32:19-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 898] [Cited by in RCA: 911] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 5. | Jalan R, Sen S, Williams R. Prospects for extracorporeal liver support. Gut. 2004;53:890-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 54] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Stange J, Mitzner S, Ramlow W, Gliesche T, Hickstein H, Schmidt R. A new procedure for the removal of protein bound drugs and toxins. ASAIO J. 1993;39:M621-M625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Rodríguez-Gaspar M, Santolaria F, Jarque-López A, González-Reimers E, Milena A, de la Vega MJ, Rodríguez-Rodríguez E, Gómez-Sirvent JL. Prognostic value of cytokines in SIRS general medical patients. Cytokine. 2001;15:232-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 64] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Mullin EJ, Metcalfe MS, Maddern GJ. Artificial liver support: potential to retard regeneration. Arch Surg. 2004;139:670-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |