Published online Feb 14, 2006. doi: 10.3748/wjg.v12.i6.896
Revised: July 20, 2005
Accepted: July 28, 2005
Published online: February 14, 2006
AIM: To analyze and to compare the effects of interferon (IFN)-α, IFN-β, and IFN-γ on pancreatic stellate cell (PSC) activation in vitro and to elucidate the molecular basis of IFN action.
METHODS: PSCs were isolated from rat’s pancreatic tissue, cultured and stimulated with recombinant rat IFNs. Cell proliferation and collagen synthesis were assessed by measuring the incorporation of 5-bromo-2’-deoxyuridine (BrdU) into DNA and [3H]-proline into acetic acid-soluble proteins, respectively. Apoptotic cells were determined by FACS analysis (sub-G1 peak method). Exhibition of the myofibroblastic PSC phenotype was monitored by immunoblot analysis of α-smooth muscle actin (α-SMA) expression. To assess the activation of signal transducer and activator of transcription (STAT), Western blots using phospho-STAT-specific antibodies were performed. In studies on STAT1 function, expression of the protein was inhibited by siRNA.
RESULTS: IFN-β and IFN-γ, but not IFN-α significantly diminished PSC proliferation and collagen synthesis. IFN-γ was the only IFN that clearly inhibited α-SMA expression. Under the experimental conditions used, no enhanced rate of apoptotic cell death was observed in response to any IFN treatment. IFN-β and IFN-γ induced a strong increase of STAT1 and STAT3 tyrosine phosphorylation, while the effect of IFN-α was much weaker. Inhibition of STAT1 expression with siRNA was associated with a significantly reduced growth-inhibitory effect of IFN-γ.
CONCLUSION: IFN-β and particularly IFN-γ display inhibitory effects on PSC activation in vitro and should be tested regarding their in vitro efficiency. Growth inhibition by IFN-γ action requires STAT1.
- Citation: Baumert JT, Sparmann G, Emmrich J, Liebe S, Jaster R. Inhibitory effects of interferons on pancreatic stellate cell activation. World J Gastroenterol 2006; 12(6): 896-901
- URL: https://www.wjgnet.com/1007-9327/full/v12/i6/896.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i6.896
Pancreatic stellate cells (PSCs) are the main source of extracellular matrix (ECM) proteins in pancreatic fibrosis[1-3], a common feature of chronic pancreatitis and pancreatic cancer[4,5]. They are closely related with hepatic stellate cells (HSCs), the key effector cells in liver fibrosis[6]. In response to pro-fibrogenic cytokines and ethanol metabolites, PSCs exhibit a myofibroblastic phenotype that is characterized by the expression of α-smooth muscle actin (α-SMA), proliferative activity, a loss of the typical retinoid-containing fat droplets, and enhanced ECM synthesis[3,7]. Similar phenotypic changes, considered as PSC activation, are induced when isolated PSCs are plated on cell-culture dishes[1,2,8-10]. The molecular principles of PSC activation in vivo and in vitro have been extensively analyzed in the past few years. Studies indicate that platelet-derived growth factor (PDGF) exerts strong mitogenic effects on PSCs, while transforming growth factor-beta (TGF-β) has been suggested as the most potent stimulator of ECM synthesis[11,12]. Furthermore, intracellular signal transduction pathways mediating PSC activation have, in part, been deciphered. Thus, we and others have recently shown that mitogen-activated protein kinases are key mediators of activation signals[13-17]. In contrast, natural antagonists of PSC activation have not been systematically studied so far. Their elucidation, however, may be helpful for the development of antifibrotic therapies.
Interferons (IFNs) are multifunctional cytokines that block viral infection, modulate immune as well as inflammatory responses, and inhibit cell proliferation[18]. IFN-α is an effective drug for the treatment of patients with chronic hepatitis B or C associated with liver fibrosis[19,20]. Recent studies suggest that IFNs not only block virus replication but also directly affect key functions of activated HSCs. Thus, IFN-γ inhibits HSC survival, cell proliferation and collagen synthesis[21-23]. The effects of IFNs on PSCs are largely unknown.
IFNs exert their biological effects on target cells by binding to cell surface receptors with specificity for either type I interferons (IFN-α, -β, and -ω) or type II interferon IFN-γ[18,24]. In the transduction of signals from activated IFN-receptors to the nuclei, tyrosine kinases of the Janus family (JAKs) and signal transducer and activator of transcription (STAT) transcription factors play a key role[18,25]. IFN-α activates the JAK family proteins (JAK1 and TYK2) as well as the ISGF3 complex (consisting of STAT1, 2 and the IFN regulatory family protein p48)[26]. Although tremendous progress has been made, the molecular mechanisms underlying the growth-inhibitory action of these cytokines are only partially characterized so far.
This study was to analyze the biological effects of IFN-α, -β and -γ on PSCs and to study the molecular determinants of IFN efficiency.
The enhanced chemiluminescence (ECL) plus kit, horseradish-peroxidase labeled antibodies and [3H]-proline were purchased from Amersham Biosciences (Freiburg, Germany), the phospho-STAT1 and STAT3 antibodies from New England Biolabs (Frankfurt, Germany), and anti-STAT1 and STAT3 protein antibodies from Santa Cruz Biotechnologies (Santa Cruz, CA, USA). Hank’s buffered salt solution (HBSS), Iscove’s modified Dulbecco’s medium (IMDM) and all supplements for cell culture were obtained from Biochrom (Berlin, Germany), Nycodenz from Nycomed (Oslo, Norway), simvastatin from Merck Biosciences (Schwalbach, Germany) and the recombinant rat interferons from R&D Systems (Minneapolis, MN, USA). Ascorbat, β-aminoproprionitrile and the α-SMA antibody as well as standard laboratory chemicals were from Sigma-Aldrich (St. Louis, MO, USA).
PSCs were isolated from the pancreas of male LEW.1W inbred rats by collagenase digestion of the organ and Nycodenz (120 g/L) density gradient centrifugation as previously described[13]. PSCs collected from the top of the gradient were washed and resuspended in IMDM supplemented with 170 g/L fetal calf serum (FCS),
10 mL/L non-essential amino acids (dilution of a 100× stock solution), 105 U/L penicillin and 100 mg/L streptomycin. The cells were cultured at 37 °C in a
50 mL/L CO2 humidified atmosphere. All experiments were performed with cells growing in primary culture, or depending on the experimental settings, with cells of the first passage. If replating of the cells was required, PSCs were harvested by trypzination on d 7 after isolation and recultured at equal seeding densities.
To quantitate cell proliferation, incorporation of 5-bromo-2’-deoxyuridine (BrdU) into newly synthesized DNA was measured using the BrdU labeling and enzyme-linked immunosorbent assay kit (Roche Diagnostics). Therefore, cells were plated in 96-well plates in complete culture medium supplemented with IFN as indicated. After 48 h, BrdU labeling was initiated by adding labeling solution at a final concentration of 20 mL/L. After another 16 h, labeling was stopped, and BrdU uptake was measured according to the manufacturer’s instructions.
Cells were grown to about 80% confluency and then incubated for 48 h in complete culture medium with or without IFN as indicated. Then the cells were harvested by trypzination, washed twice with PBS and resuspended in 2 mL ice-cold 70% ethanol for at least 12 h at
-20 °C, washed again twice with PBS and incubated for 1 h in 400 µL PBS containing 1 mL/L Tween 20 and
1 g/L RNase at room temperature. After the addition of 50 µg propidium iodide/106 cells, samples were subjected to cytofluorometric analysis using an FACScan (Becton Dickinson) and the CellQuest program. Ten thousand events were measured for each sample and the data were stored in list mode for further analysis.
Collagen synthesis was assessed through the quantification of [3H]-proline incorporation into acetic acid-soluble proteins. Therefore, cells were plated in 12-well plates and grown to subconfluency. Afterwards, they were cultured for 48 h in complete culture medium supplemented with 2.5 mCi/L [3H]-proline (48 Ci/mmol), 50 mg/L ascorbate, 50 mg/L β-aminoproprionitrile and IFN as indicated. All further steps were performed essentially as previously described[27]. Raw data of [3H]-proline incorporation were normalized on the basis of absolute cell counts determined by trypan blue staining of PSCs cultured in parallel under identical conditions, except that no [3H]-proline was added.
Protein extracts of PSCs (pretreated as indicated) were prepared, adjusted to identical protein concentrations and subjected to immunoblot analysis as previously described[13]. Briefly, proteins (15 µg/sample) were separated by SDS-polyacrylamide gel electrophoresis and blotted onto nitrocellulose membrane. Next, the filters were blocked with 1% bovine serum albumin (Sigma-Aldrich) and incubated with the indicated antibodies (diluted according to the manufacturer’s instructions) overnight at 4 °C. After a final incubation with a horseradish-peroxidase labeled anti-rabbit or anti-mouse Ig antibody for 2 h at room temperature, blots were developed using ECL. For reprobing with additional antibodies, membranes were stripped by incubation in stripping buffer
(62.5 mmol/L Tris-HCl, pH 6.7, 20 g/L SDS, 100 mmol/L 2-mercaptoethanol) at 50 °C for 30 min.
STAT1 siRNA was purchased from Qiagen (Hilden, Germany) and applied according to the manufacturer’s protocol for the transfection of adherent cells, using a 1:6 ratio of siRNA to RNAsFect Reagent. Efficiency of siRNA treatment was monitored by analyzing the STAT1 protein level at different times after siRNA application. To study the effects of STAT1 siRNA on PSC growth, cells growing in 96-well plates were pretreated with siRNA at 100 nmol/L for 24 h before IFN-γ was added as indicated. After 48 h of incubation, DNA synthesis was quantitated by the determination of BrdU incorporation into DNA as described above. In all the experiments, a non-silencing siRNA oligonucleotide was included as a negative control.
Results were expressed as mean±SE for the indicated number of separate cultures per experimental protocol. Statistical significance was checked using Wilcoxon’s rank sum test. P < 0.05 was considered statistically significant.
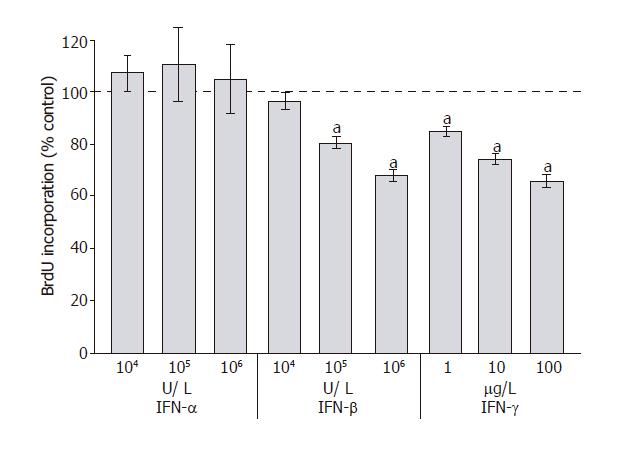
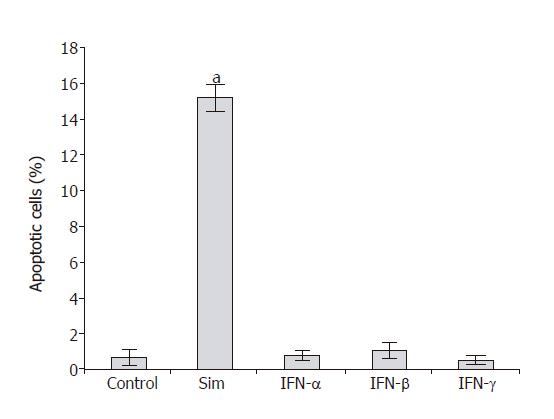
To analyze the effects of IFN-α, IFN-β and IFN-γ on PSC proliferation, DNA synthesis after IFN-pretreatment for 48 h was measured (Figure 1). Both IFN-β and IFN-γ inhibited DNA synthesis significantly and in a dose-dependent manner, causing more than 30% reduction at the highest concentration tested. In contrast, IFN-α even at the concentration of 106 U/L had no effect on PSC growth. To study if the inhibition of DNA synthesis was in fact due to the induction of apoptosis, an FACS analysis was performed (Figure 2). Under the experimental conditions tested, none of IFN-α, IFN-β or IFN-γ induced a significant increase of the sub-G1 fraction (representing apoptotic cells). In contrast, PSCs treated with simvastatin significantly accumulated in the sub-G1 fraction, confirming our previous results of pro-apoptotic effects of hydroxymethylglutaryl coenzyme A reductase inhibitors[28].
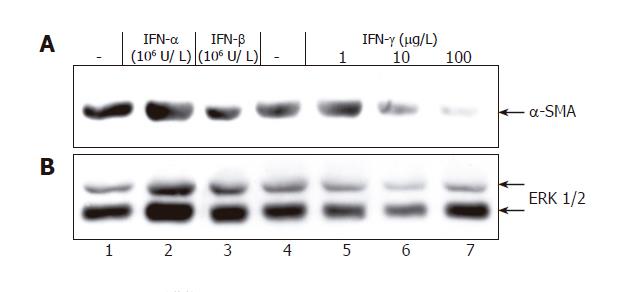
Next, we asked how IFNs affect the myofibroblastic phenotype of activated PSCs. IFN-γ incubation for
9 d (starting immediately after isolation) was associated with a dose-dependent reduction of α-SMA protein expression (Figure 3), while the effect of IFN-β (at
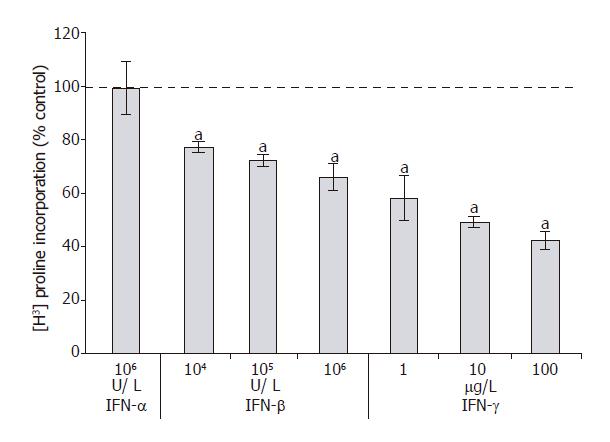
106 U/L) remained questionable and IFN-α did not display any inhibitory effect at all. Further studies revealed that IFN-γ (at ≥1 µg/L) and IFN-β (at ≥104 U/L), but not IFN-α (at 106 U/L) significantly diminished collagen synthesis by PSCs (Figure 4). A further increase of IFN-γ and IFN-β concentrations had no additional effect (data not shown).
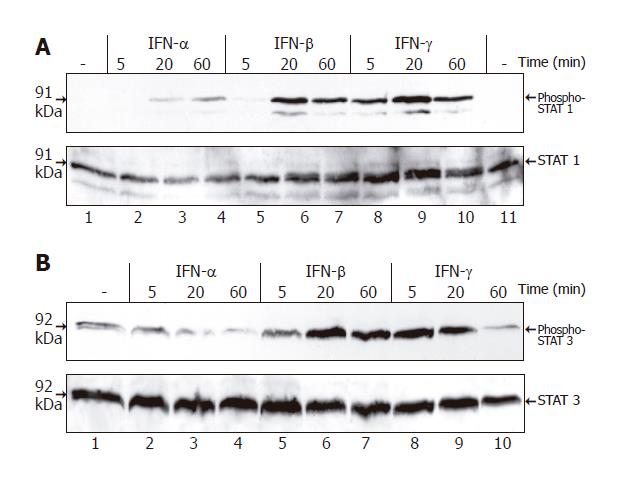
To correlate the biological effects of the three IFNs with their molecular action, we analyzed STAT protein activation in IFN-stimulated PSCs (Figure 5). IFN-β and IFN-γ induced a rapid and strong increase of STAT1 and STAT3 tyrosine phosphorylation, while IFN-α was much less effective.
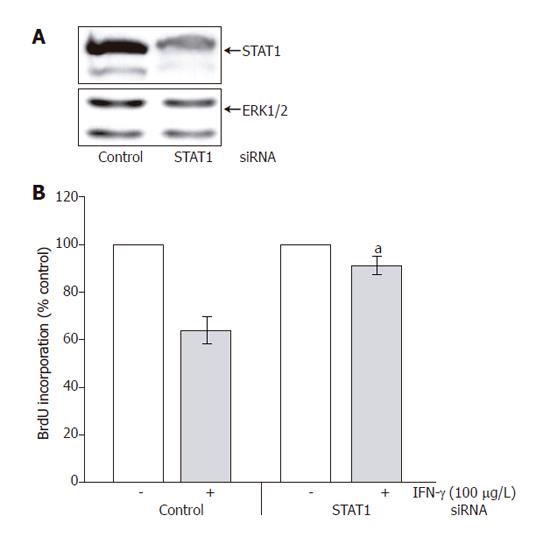
In subsequent experiments, we further studied the role of STAT1 in the mediation of IFN-γ effects on PSC growth. As shown in Figure 6A (upper panel), STAT1 siRNA treatment caused a marked decrease of the STAT1 protein level. Expression of ERK 1 and 2 remained unchanged (lower panel), suggesting that the siRNA inhibited STAT1 expression in a specific manner. Downregulation of the STAT1 protein level was associated with a significant attenuation of the growth-inhibitory effect of IFN-γ (Figure 6B).
Chronic pancreatitis is associated with an extended fibrosis that contributes to the development of an exocrine and endocrine insufficiency of the gland. Excessive deposition of connective tissue is also typical of pancreatic cancer and has been implicated in the acceleration of tumor progression[29]. In both cases, activated PSCs have been identified as the principle ECM-producing cell type[3,7,30]. PSCs are therefore considered as a promising target for the development of adjuvant therapies aimed at inhibiting pancreatic fibrogenesis.
The results of this study showed that IFNs displayed direct inhibitory effects on key effector functions of activated PSCs. While both IFN-γ and IFN-β diminished PSC proliferation and collagen synthesis, only IFN-γ efficiently reduced the expression of α-SMA, an indicator of myofibroblastic transdifferentiation. The effects of IFN-α remained insignificant. Whether the latter observation could reflect a peculiarity of the in vitro model used in this study, or a general phenomenon of PSC biology, is currently unclear.
The findings described here are largely in agreement with the results of studies on hepatic stellate cells which also showed direct, but distinct inhibitory effects of different IFNs on stellate cell activation. Shen et al[21] observed that HSC proliferation is attenuated by IFN-β and IFN-γ, but not by IFN-α. On the other hand, under the experimental conditions used in this study no significant effects of IFNs on cell survival were detected, while in HSCs pro-apoptotic effects of IFN-γ and an anti-apoptotic action of IFN-α have been reported[23].
Targeted gene disruption studies have shown that the transcription factor STAT1 plays an essential, non-redundant role in IFN-mediated innate immunity to viral diseases[31,32]. Analyzing possible functions of STAT proteins in PSCs, we found a correlation between the efficiency of the different IFNs to inhibit stellate cell functions and their potential to activate STAT1 (as well as STAT3). Further experiments using an siRNA approach to block STAT1 expression revealed a direct involvement of the transcription factor in PSC growth inhibition by IFN-γ. The precise functions of STAT3 in PSCs remain to be characterized.
Previous studies in our laboratory have shown that IFN-γ in PSCs stimulates the expression of interleukin (IL)-15. In a co-culture model of rat PSCs and lymphocytes, a suppression of spontaneous lymphocyte apoptosis has been observed, which is at least in part mediated by IL-15[33]. Together, these data and the results of this study suggest a complex role of IFN-γ in the modulation of pancreatic inflammation and fibrosis. It is conceivable that IFN-γ displays distinct net effects on the progression of chronic pancreatitis, depending on the activation of auto-reactive lymphocytes and possibly the stage of the disease. To further elucidate the effects of IFN-γ on pancreatic fibrogenesis, in vivo studies using animal models of pancreatic fibrosis are required.
In summary, the discovery of direct inhibitory IFN-effects on PSC activation encourages further studies with respect to IFN efficiency in chronic pancreatitis. Currently, we are also focusing on a systematic evaluation of IFN target genes in stellate cells.
We gratefully acknowledge the excellent technical assistance of Mrs. Helga Schulze and Mrs. Katja Bergmann.
S- Editor Wang XL and Guo SY L- Editor Elsevier HK E- Editor Kong LH
| 1. | Bachem MG, Schneider E, Gross H, Weidenbach H, Schmid RM, Menke A, Siech M, Beger H, Grunert A, Adler G. Identification, culture, and characterization of pancreatic stellate cells in rats and humans. Gastroenterology. 1998;115:421-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 744] [Cited by in RCA: 798] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 2. | Apte MV, Haber PS, Applegate TL, Norton ID, McCaughan GW, Korsten MA, Pirola RC, Wilson JS. Periacinar stellate shaped cells in rat pancreas: identification, isolation, and culture. Gut. 1998;43:128-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 719] [Cited by in RCA: 716] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 3. | Haber PS, Keogh GW, Apte MV, Moran CS, Stewart NL, Crawford DH, Pirola RC, McCaughan GW, Ramm GA, Wilson JS. Activation of pancreatic stellate cells in human and experimental pancreatic fibrosis. Am J Pathol. 1999;155:1087-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 323] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 4. | Longnecker DS. Pathology and pathogenesis of diseases of the pancreas. Am J Pathol. 1982;107:103-121. [PubMed] |
| 5. | Etemad B, Whitcomb DC. Chronic pancreatitis: diagnosis, classification, and new genetic developments. Gastroenterology. 2001;120:682-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 859] [Cited by in RCA: 758] [Article Influence: 31.6] [Reference Citation Analysis (1)] |
| 6. | Gressner AM, Bachem MG. Molecular mechanisms of liver fibrogenesis--a homage to the role of activated fat-storing cells. Digestion. 1995;56:335-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 170] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 7. | Apte MV, Wilson JS. Stellate cell activation in alcoholic pancreatitis. Pancreas. 2003;27:316-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 79] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | Apte MV, Haber PS, Darby SJ, Rodgers SC, McCaughan GW, Korsten MA, Pirola RC, Wilson JS. Pancreatic stellate cells are activated by proinflammatory cytokines: implications for pancreatic fibrogenesis. Gut. 1999;44:534-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 474] [Cited by in RCA: 484] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 9. | Mews P, Phillips P, Fahmy R, Korsten M, Pirola R, Wilson J, Apte M. Pancreatic stellate cells respond to inflammatory cytokines: potential role in chronic pancreatitis. Gut. 2002;50:535-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 291] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 10. | Apte MV, Phillips PA, Fahmy RG, Darby SJ, Rodgers SC, McCaughan GW, Korsten MA, Pirola RC, Naidoo D, Wilson JS. Does alcohol directly stimulate pancreatic fibrogenesis Studies with rat pancreatic stellate cells. Gastroenterology. 2000;118:780-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 182] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 11. | Luttenberger T, Schmid-Kotsas A, Menke A, Siech M, Beger H, Adler G, Grünert A, Bachem MG. Platelet-derived growth factors stimulate proliferation and extracellular matrix synthesis of pancreatic stellate cells: implications in pathogenesis of pancreas fibrosis. Lab Invest. 2000;80:47-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 151] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 12. | Kruse ML, Hildebrand PB, Timke C, Folsch UR, Schmidt WE. TGFbeta1 autocrine growth control in isolated pancreatic fibroblastoid cells/stellate cells in vitro. Regul Pept. 2000;90:47-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 47] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Jaster R, Sparmann G, Emmrich J, Liebe S. Extracellular signal regulated kinases are key mediators of mitogenic signals in rat pancreatic stellate cells. Gut. 2002;51:579-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 114] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 14. | Masamune A, Kikuta K, Satoh M, Satoh A, Shimosegawa T. Alcohol activates activator protein-1 and mitogen-activated protein kinases in rat pancreatic stellate cells. J Pharmacol Exp Ther. 2002;302:36-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 85] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | McCarroll JA, Phillips PA, Park S, Doherty E, Pirola RC, Wilson JS, Apte MV. Pancreatic stellate cell activation by ethanol and acetaldehyde: is it mediated by the mitogen-activated protein kinase signaling pathway. Pancreas. 2003;27:150-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 63] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 16. | Masamune A, Satoh M, Kikuta K, Sakai Y, Satoh A, Shimosegawa T. Inhibition of p38 mitogen-activated protein kinase blocks activation of rat pancreatic stellate cells. J Pharmacol Exp Ther. 2003;304:8-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 77] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 17. | Masamune A, Kikuta K, Suzuki N, Satoh M, Satoh K, Shimosegawa T. A c-Jun NH2-terminal kinase inhibitor SP600125 (anthra[1,9-cd]pyrazole-6 (2H)-one) blocks activation of pancreatic stellate cells. J Pharmacol Exp Ther. 2004;310:520-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Stark GR, Kerr IM, Williams BR, Silverman RH, Schreiber RD. How cells respond to interferons. Annu Rev Biochem. 1998;67:227-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3048] [Cited by in RCA: 3080] [Article Influence: 114.1] [Reference Citation Analysis (0)] |
| 19. | Nguyen MH, Wright TL. Therapeutic advances in the management of hepatitis B and hepatitis C. Curr Opin Infect Dis. 2001;14:593-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Schuppan D, Krebs A, Bauer M, Hahn EG. Hepatitis C and liver fibrosis. Cell Death Differ. 2003;10 Suppl 1:S59-S67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 157] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 21. | Shen H, Zhang M, Minuk GY, Gong Y. Different effects of rat interferon alpha, beta and gamma on rat hepatic stellate cell proliferation and activation. BMC Cell Biol. 2002;3:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Saile B, Eisenbach C, Dudas J, El-Armouche H, Ramadori G. Interferon-gamma acts proapoptotic on hepatic stellate cells (HSC) and abrogates the antiapoptotic effect of interferon-alpha by an HSP70-dependant pathway. Eur J Cell Biol. 2004;83:469-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Uzé G, Lutfalla G, Mogensen KE. Alpha and beta interferons and their receptor and their friends and relations. J Interferon Cytokine Res. 1995;15:3-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 185] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 24. | Rane SG, Reddy EP. Janus kinases: components of multiple signaling pathways. Oncogene. 2000;19:5662-5679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 329] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 25. | Ihle JN. Cytokine receptor signalling. Nature. 1995;377:591-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 977] [Cited by in RCA: 970] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 26. | Jaster R, Hilgendorf I, Fitzner B, Brock P, Sparmann G, Emmrich J, Liebe S. Regulation of pancreatic stellate cell function in vitro: biological and molecular effects of all-trans retinoic acid. Biochem Pharmacol. 2003;66:633-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Jaster R, Brock P, Sparmann G, Emmrich J, Liebe S. Inhibition of pancreatic stellate cell activation by the hydroxymethylglutaryl coenzyme A reductase inhibitor lovastatin. Biochem Pharmacol. 2003;65:1295-1303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | Bachem MG, Schünemann M, Ramadani M, Siech M, Beger H, Buck A, Zhou S, Schmid-Kotsas A, Adler G. Pancreatic carcinoma cells induce fibrosis by stimulating proliferation and matrix synthesis of stellate cells. Gastroenterology. 2005;128:907-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 460] [Cited by in RCA: 505] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 29. | Apte MV, Park S, Phillips PA, Santucci N, Goldstein D, Kumar RK, Ramm GA, Buchler M, Friess H, McCarroll JA. Desmoplastic reaction in pancreatic cancer: role of pancreatic stellate cells. Pancreas. 2004;29:179-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 479] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 30. | Meraz MA, White JM, Sheehan KC, Bach EA, Rodig SJ, Dighe AS, Kaplan DH, Riley JK, Greenlund AC, Campbell D. Targeted disruption of the Stat1 gene in mice reveals unexpected physiologic specificity in the JAK-STAT signaling pathway. Cell. 1996;84:431-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1278] [Cited by in RCA: 1300] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 31. | Durbin JE, Hackenmiller R, Simon MC, Levy DE. Targeted disruption of the mouse Stat1 gene results in compromised innate immunity to viral disease. Cell. 1996;84:443-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1205] [Cited by in RCA: 1219] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 32. | Sparmann G, Glass A, Brock P, Jaster R, Koczan D, Thiesen HJ, Liebe S, Emmrich J. Inhibition of lymphocyte apoptosis by pancreatic stellate cells: impact of interleukin-15. Am J Physiol Gastrointest Liver Physiol. 2005;289:G842-G851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |