Published online Feb 14, 2006. doi: 10.3748/wjg.v12.i6.874
Revised: July 23, 2005
Accepted: July 29, 2005
Published online: February 14, 2006
AIM: To investigate the anti-tumor activity of ursolic acid (UA) and its derivatives isolated from Aralia decaisneana on hepatocellular carcinoma both in vitro and in vivo.
METHODS: In vivo cytotoxicity was first screened by 3-[4,5-dimethylthiazol-2-yl]-2, 5-diphenyltetrazolium bromide (MTT) assay. Morphological observation, DNA ladder, flow cytometry analysis, Western blot and real time PCR were employed to elucidate the cytotoxic mechanism of UA. Implanted mouse hepatoma H22 was used to evaluate the growth inhibitory effect of UA in vivo.
RESULTS: UA could significantly inhibit the proliferation of HepG2 and its drug-resistance strain, R-HepG2 cells, but had no inhibitory effect on primarily cultured normal mouse hepatocytes whereas all the six derivatives of UA could not inhibit the growth of all tested cell lines. Further study on mechanism demonstrated that apoptosis and G0/G1 arrest were involved in the cytotoxicity and cleavage of poly-(ADP-ribose)-polymerase (PARP). Downregulation of cyclooxygenase-2 (COX-2) protein and upregulation of heat shock protein (HSP) 105 mRNA correlated to the apoptosis of HepG2 cells treated with UA. In addition, UA also could inhibit the growth of H22 hepatoma in vivo.
CONCLUSION: UA is a promising anti-tumor agent, but further work needs to be done to improve its solubility.
- Citation: Tian Z, Lin G, Zheng RX, Huang F, Yang MS, Xiao PG. Anti-hepatoma activity and mechanism of ursolic acid and its derivatives isolated from Aralia decaisneana. World J Gastroenterol 2006; 12(6): 874-879
- URL: https://www.wjgnet.com/1007-9327/full/v12/i6/874.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i6.874
Hepatocellular carcinoma is one of the most common malignant neoplasms worldwide and one of the leading causes of malignancy-related death in China[1,2]. Its therapy in clinic is still a big challenge.
Ursolic acid (UA) is a versatile compound, which possesses anti-cancer[3,4], anti-inflammatory[5,6], anti-HIV[7] and immunomodulatory effects[8]. There is a growing interest in the anti-tumor activities of UA. UA could act on almost all steps in the whole cancer process: initiation, promotion, progression, and metastasis. Multiple lines of evidence indicate that UA is a promising chemo-preventive agent both in vivo and in vitro. UA could inhibit Epstein-Barr virus activation induced by 12-O-tetradecanoylphorbol-13-acetate in Raji cells and two-stage mouse skin tumor promotion[9,10]. The mechanism involved might associate with the inhibition of AP-1-mediated induction of COX-2 by disrupting PKC signal transduction pathway[11]. UA could inhibit the proliferation of various cancerous cell lines by inhibiting DNA polymerase and topoisomerase[12], inducing apoptosis through proteolytic activation of caspase-3 and/or other similar caspases[3]. It was also reported that UA has anti-invasion and anti-metastasis activity by inhibiting nuclear factor-kappa B (NF-κB) activation, down-regulating COX-2 and matrix metalloproteinase 9[13].
Besides its cytotoxicity to hepatoma, it was reported that UA can exert its hepatoprotective action in mice and rats[14-16].The reason for the phenomenon is worth studying. Moreover, the growth inhibitory activity of UA on hepatocellular carcinoma in vivo is still unknown.
In the present study, UA and its six derivatives isolated from the bark of Aralia decaisneana were used to treat rheumatism, lumbago, hepatitis, nephritis, and diabetes mellitus in Chinese folk medicine. The cytotoxicity of these UA compounds to HepG2, its drug-resistance strain R-HepG2 and primarily cultured normal mouse and rat hepatocytes were examined in order to find their anti-hepatoma efficiency on both parental and drug-resistant hepatocellular carcinoma as well as their low toxicity. Morphological observation, DNA ladder, cell cycle analysis, Western blot and real time PCR were performed to elucidate the cytotoxic mechanism of UA on HepG2 cells. Mice bearing murine hepatoma H22 were used to investigate the growth inhibitory effect of UA in vivo.
HepG2 (ATCC) cells were maintained in RPMI 1640 (Gibco) containing 10% FBS (Gibco), 2 mg/mL sodium bicarbonate, 100 µg/mL penicillin sodium salt and 100 µg/mL streptomycin sulfate. R-HepG2 (City University of Hong Kong) was maintained in the presence of 1.2 µmol/L doxorubicin (Sigma). Hepatocytes were isolated from normal Kunming mice (Experimental Animal Centre of Zhongshan Medical University) and SD rats (Experimental Animal Centre of Zoology, Chinese Academy of Sciences) with enzymatic perfusion technique as previously described[17]. Logarithmically growing cells were used for all the experiments.
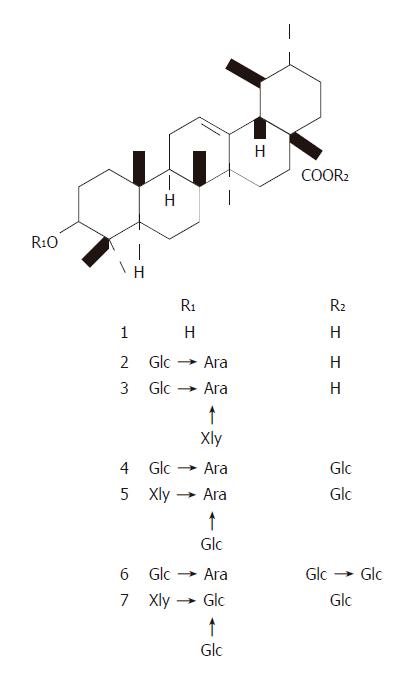
UA and its derivatives (Figure 1) were isolated from the bark of Aralia decaisneana by silica gel column chromatography and their structures were identified by spectral analysis[18]. UA was dissolved in DMSO at the concentration of 100 and 10 mmol/L in cellular and in vivo experiments, respectively and diluted in tissue culture medium and saline before use. Six UA derivatives were directly dissolved in tissue culture medium.
HepG2 and R-HepG2 cells (1.5×104) as well as mouse and rat hepatocytes (8×103) were seeded in 96-well plates and treated with the compounds at various concentrations (3.125-100 µmol/L). Then the cells were incubated at 37 °C in an atmosphere containing 50 mL/L CO2 for 48 h followed by MTT assay. IC50 of the tested compounds on different cell lines were obtained from the concentration–effect curves.
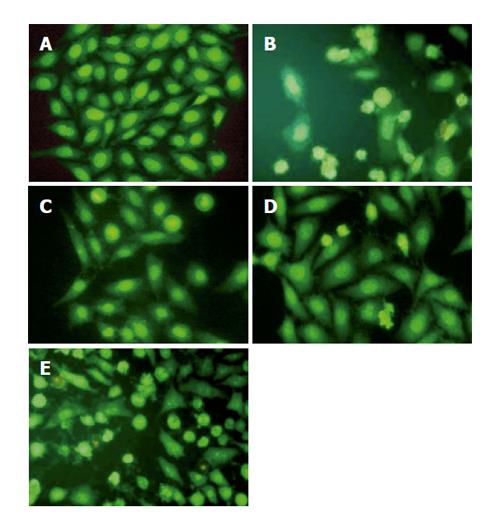
HepG2 cells were cultured in 3.5-cm dishes. UA was added to the medium at 20 μmol/L for 6, 12, and 24 h. A harringtonin-(20 μmol/L, 24 h) and vehicle-(0.1% DMSO) treated sample was regarded as a positive and negative control, respectively. After the treatment, all the cultures were incubated at 37 °C in an atmosphere containing 50 mL/L CO2 for the indicated time. Photographs were taken under an inverted Leica fluorescence 40×10 microscope after acridine orange (AO)/ethidium bromide (EB) staining.
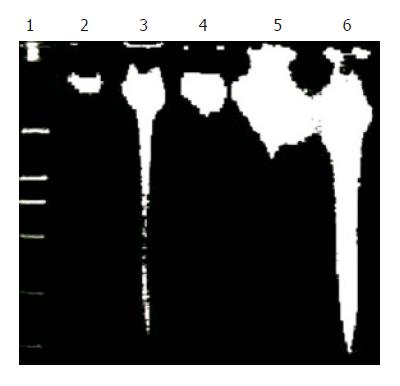
HepG2 cells were grown to 70–80% confluence and exposed to 20 µmol/L UA or harringtonin. Then the cultures were incubated at 37 °C in an atmosphere containing 50 mL/L CO2 and collected at 0, 6, 12, and 24 h. Their genomic DNA was extracted and analyzed by gel electrophoresis[19].
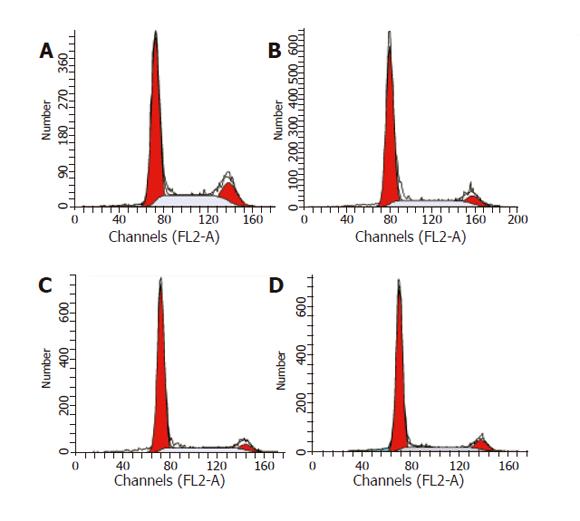
Flow cytometry analysis was used to evaluate cell cycle distribution of HepG2 cells. HepG2 cells were treated with 20 µmol/L UA for 0, 6, 12, and 24 h, respectively, collected and fixed in 70% cold ethanol (–20 °C) overnight. After being washed twice with PBS, the cells were resuspended in PBS. RNA in the fixed cells was digested using RNase A (0.5 mg/mL) at 37 °C for 1 h. Finally, the cells were stained with 2.5 μg/mL propidium iodide (PI). The DNA content of the cells was then analyzed with an FACSCalibur instrument (Becton-Dickinson) at excitation 488/emission 600 nm. Data were analyzed by cell cycle distribution software (ModFit LT version 2.0, Verity Software House, USA).
After the treatment, cells were washed thrice with ice-cold PBS and collected with lysis buffer. Protein determination, SDS-PAGE and transfer were performed as previously described[17]. Nitrocellulose membranes were then incubated with a monoclonal anti-PARP or polyclonal anti-COX-2 antiserum. Secondary antibody to IgG conjugated to horseradish peroxidase was used. The blots were probed with the ECL Western blot detection system according to the manufacturer’s instructions.
Total RNA was isolated from HepG2 cells using Trizol reagent according to the standard protocol and used to generate cDNA in each sample using the SuperScript II reverse transcriptase with oligo (dT) primers. Aliquots of total cDNA were diluted and added into each PCR reaction mixture together with 0.5 µmol/L of forward and reverse primers of HSP 105 (F: CAGGATCCAGTTGTACGTGCTC; R: TGATGTGGAGGTTCAGCACC, 197 bp) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH, F: ATCAGCAATGCCTCCTGCA; R: CCTGCTTCACCACCTTCTTGA, 355 bp) (Shanghai Biotech Engineering Service Limited Company). PCR amplification was performed on a GeneAmp 9700 PCR System (ABI). After denaturation of cDNA at 94 °C for 3 min, the cycling conditions were as follows: 32 cycles consisting of denaturation at 94 °C for 30 s, annealing at 59 °C for 30 s, and extension at 72 °C for 1 min. The PCR products were analyzed by agarose gel electrophoresis and Bio-Rad Chemi Doc. The mRNA expression levels were normalized to the expression of a housekeeping gene GAPDH. SYBR-Green-I fluorescence labeling method was used in real-time quantitative RT-PCR amplification on an ABI Prism 7000 SDS and analyzed with its software according to the standard protocol and manufacturer’s instructions[20]. The 2–ΔΔCT method was used in data analysis based on similar (<10%) amplification efficiency of the target and reference genes, which was achieved by the determination of ΔCT variations with template dilution[21]. GAPDH was used as a reference gene for internal control.
Male CD-1 (ICR) mice (Beijing Vital Laboratory Animal Technology Company) weighing 20–22 g were used for the implantation of hepatoma H22 (s.c.) which was maintained by weekly i.p. passages in CD-1 (ICR) mice, 0.2 mL ascites of 1:6 dilution from tumor-bearing mice 7 days after the tumor inoculation was implanted (s.c.) into the armpit region of the mice. Eight mice were treated i.p. with either UA or vehicle (1% DMSO in saline) once a day for 10 days 24 h after the tumor inoculation. Cyclophosphamide (10 mg/kg b.w.) was used as positive control. Tumor inhibition rate (TIR) was derived from (1–T/C)×100, where T is the mean tumor weight of the UA-treated group and C is the mean tumor weight of the negative control group.
Student’s t-test was used in all the experiments. P<0.05 was considered statistically significant.
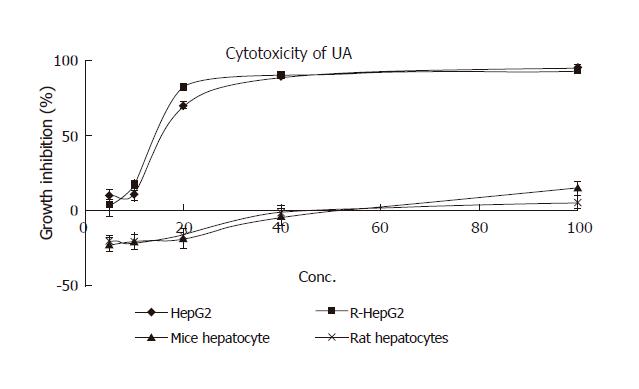
Cytotoxicity of UA and its derivatives was determined by MTT assay. UA was demonstrated to have similar anti-proliferation activity on HepG2 and R-HepG2 cells in a concentration-dependent manner (Figure 2) with its IC50 value being 18 and 15 μmol/L, respectively, whereas it exhibited less growth inhibitory activity on primarily cultured normal mouse and rat hepatocytes and the IC50 value could not be detected at the tested concentration. On the contrary, six UA derivatives did not show any cytotoxicity to all the tested cells, while they possessed better solubility in water than UA.
HepG2 cells treated with harringtonin and 20 μmol/L UA showed similar and significant changes (Figure 3).
Chromatin aggregation, nuclear, and cytoplasmic condensation only could be seen in a few cells after being treated with UA for 6 and 12 h. Partition of cytoplasm and nuclei into the membrane bound-vesicles (apoptotic bodies) was observed in the cells treated with harringtonin and UA for 24 h. At the same time, apoptotic cells were found at this time point.
DNA ladder was used to prove the apoptosis of HepG2 cells induced by UA. After treatment with 20 μmol/L UA for different time points, evident DNA ladder was detected at 24 h on HepG2 cells treated with UA and harringtonin (Figure 4), indicating the apoptosis observed in morphological inspection.
After being exposed to 20 μmol/L UA for 0, 6, 12, and 24 h, respectively, the cell cycle progression of HepG2 cells showed evident changes (Figure 5). UA caused significant G0/G1 arrest with a concomitant decrease of cell population in S and G2/M phases. The distribution of cell cycle in G0/G1, S and G2/M phases was 57.80%, 27.24%, and 14.96% at 0 h; 67.78%, 22.92%, and 9.3% at 6 h; 73.73%, 19.59%, and 6.69% at 12 h; 74.31%, 15.69%, and 10.0% at 24 h, respectively. When treated with UA for 12 and 24 h, higher percentage of cell cycle distribution in G0/G1 phase was detected than that at 6 h.
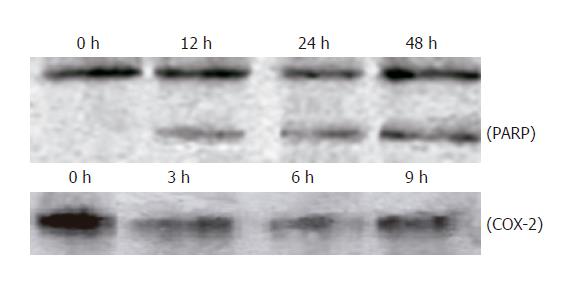
Exposure to 20 μmol/L UA for 0, 12, 24, and 48 h, respectively, PARP was cleaved into 89- and 24-ku fragments, indicating that the apoptosis of HepG2 cells induced by UA was involved in caspase activation. After HepG2 cells were treated with UA for 0, 3, 6, and 9 h, respectively, COX-2 expression was significantly inhibited (Figure 6).
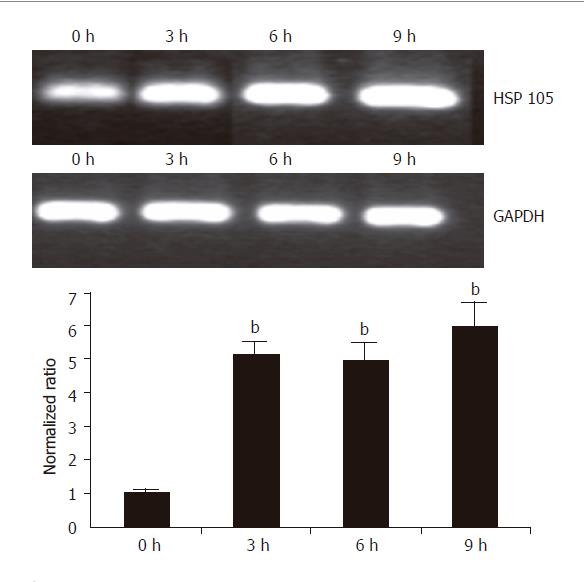
HSP 105 belongs to HSP 70 super family and is closely related with apoptosis. The amplification efficiency of target and reference genes HSP 105 and GAPDH was quite similar (data not shown). The 2–ΔΔCt method was used in data analysis. After being treated with 20 μmol/L UA for 3, 6, and 9 h, respectively, HSP 105 mRNA expression was upregulated in a time-dependent manner both in RT-PCR and real time PCR (Figure 7).
Twenty-four hours after tumor implantation, administration of UA (30 and 15 mg/kg b.w.) and cyclophosphamide (10 mg/kg b.w.) once a day for 10 days, could significantly suppress the growth of H22 (Table 1). Significant body weight loss was observed in cyclophosphamide-treated group compared to the control group, whereas only slight body weight loss was observed in UA-treated group, suggesting that UA might possess anti-hepatoma effect in vivo with low toxicity.
| Samples | Dosage (mg/kg) | Tumor weight (g) | Growth inhibition (%) |
| Control | 2.8±1.22 | ||
| C.P. | 10 | 1.07±0.55b | 61.1 |
| UA | 30 | 1.27±0.90a | 54.6 |
| UA | 15 | 1.55±0.91a | 44.6 |
In terms of cancer treatment, chemotherapy has serious limitations, namely the lack of selectivity of active ingredients and resistance of cancer cells to these chemicals[22]. Thus, it is urgent to find new compounds that can kill both parental cancer cells and drug resistant cells and differentiate between normal and cancer cells in order to selectively kill cancerous cells with reduced toxicity. UA possesses good cytotoxicity on HepG2 and R-HepG2 cells, but no cytotoxicity to primarily cultured normal mouse and rat hepatocytes has been detected at corresponding concentrations. These results indicate that UA is a promising anti-tumor agent to parental and drug resistant HepG2 cells with low toxicity and could partially explain why UA inhibits the proliferation of hepatoma while possesses hepatoprotective activity. In addition, UA can lead to apoptosis, DNA ladder and PARP protein cleavage as well as G0/G1 cell-cycle arrest.
However, the cytotoxic mechanisms of HepG2 cells induced by UA are not completely understood. PARP is a substrate of apoptosis specific protease from the ICE-family (caspases). The 113 ku PARP is cleaved during apoptosis into 89- and 24-ku fragments which could serve as an early hallmark of apoptosis. In our study, cleaved PARP protein not only further proved apoptosis, but also it was implied that activation of caspases was involved in apoptosis of HepG2 cells treated with UA. COX-2 is an early-response gene that is highly inducible by mitogenic and inflammatory stimuli[23-25]. Multiple lines of evidence suggest that COX-2 is important in carcinogenesis and overexpressed in cancer cells[26,27]. NS-398, a representative of non-selective COX inhibitors can induce G0/G1 cell cycle arrest through inhibition of CDK2 and induction of p21 and p27[28,29]. Upregulation of p21 could further stimulate mRNA transcription of cytochrome C and caspase 3 which induces apoptosis in the end[30]. In the light of our study, UA could significantly inhibit COX-2 protein expression, suggesting that inhibition of COX-2 may correlate with increased p21 expression and subsequent activation of cytochrome C and caspase-3 in HepG2 cells treated with UA[31].
HSP 105 belonging to heat shock protein (HSP) 70 super family acts as a chaperone and plays a role in control of cell proliferation and cellular aging. It was reported that overexpression of HSP105α could enhance stress-induced apoptosis, but not necrosis in mouse embryonal F9 cells[32,33]. Some HSPs purified from murine tumors and used as vaccine are prophylactically and therapeutically effective in cancer immunotherapy models[34]. Further more, MKT-077, a cationic rhodacyanine dye analog exerts its selective toxicity to cancer cells by binding to the HSP 70 family protein mot-2 and reactivating p53 function, thus resulting in increased p53 downstream gene p21 expression[35]. Upregulation of HSP 105 and increase of p21 expression[31] may contribute to apoptosis and G0/G1 cell-cycle arrest induced by UA, which are at least partially responsible for the selectivity of UA for cancer cells.
Tumor growth inhibitory effect in mice bearing hepatoma could predict the activity of corresponding tumors in human beings. Accordingly, the anti-neoplasm action of UA on mouse hepatoma H22 indicates that it can be used to treat human hepatoma. However, few researches have been done on the pharmacokinetics of UA. We also did not monitor the concentration of UA in the blood of mice. Therefore, more attention should be paid to the active plasma concentration of UA in future.
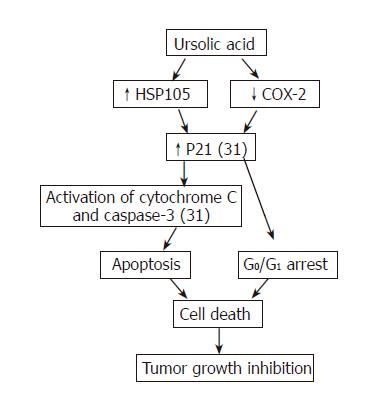
In conclusion, UA is a potential anti-hepatoma agent with reduced toxicity both in vitro and in vivo. Summary of current understanding of anti-tumor activity of UA on hepatoma is shown in Figure 8. However, poor water solubility of UA has confined its use. Although its derivatives have good water solubility, these derivatives possess no anti-neoplasm activity. More work should be done to solve this problem.
S- Editor Wang XL and Guo SY L- Editor Elsevier HK E- Editor Liu WF
| 1. | Zhu ZZ, Cong WM, Liu SF, Dong H, Zhu GS, Wu MC. Homozygosity for Pro of p53 Arg72Pro as a potential risk factor for hepatocellular carcinoma in Chinese population. World J Gastroenterol. 2005;11:289-292. [PubMed] |
| 2. | Yuan SL, Wei YQ, Wang XJ, Xiao F, Li SF, Zhang J. Growth inhibition and apoptosis induction of tanshinone II-A on human hepatocellular carcinoma cells. World J Gastroenterol. 2004;10:2024-2028. [PubMed] |
| 3. | Hollosy F, Meszaros G, Bokonyi G, Idei M, Seprodi A, Szende B, Keri G. Cytostatic, cytotoxic and protein tyrosine kinase inhibitory activity of ursolic acid in A431 human tumor cells. Anticancer Res. 2000;20:4563-4570. [PubMed] |
| 4. | Hsu HY, Yang JJ, Lin CC. Effects of oleanolic acid and ursolic acid on inhibiting tumor growth and enhancing the recovery of hematopoietic system postirradiation in mice. Cancer Lett. 1997;111:7-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 125] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 5. | Diaz AM, Abad MJ, Fernandez L, Recuero C, Villaescusa L, Silvan AM, Bermejo P. In vitro anti-inflammatory activity of iridoids and triterpenoid compounds isolated from Phillyrea latifolia L. Biol Pharm Bull. 2000;23:1307-1313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Baricevic D, Sosa S, Della Loggia R, Tubaro A, Simonovska B, Krasna A, Zupancic A. Topical anti-inflammatory activity of Salvia officinalis L. leaves: the relevance of ursolic acid. J Ethnopharmacol. 2001;75:125-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 212] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 7. | Min BS, Jung HJ, Lee JS, Kim YH, Bok SH, Ma CM, Nakamura N, Hattori M, Bae K. Inhibitory effect of triterpenes from Crataegus pinatifida on HIV-I protease. Planta Med. 1999;65:374-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Raphael TJ, Kuttan G. Effect of naturally occurring triterpenoids glycyrrhizic acid, ursolic acid, oleanolic acid and nomilin on the immune system. Phytomedicine. 2003;10:483-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 109] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 9. | Banno N, Akihisa T, Tokuda H, Yasukawa K, Higashihara H, Ukiya M, Watanabe K, Kimura Y, Hasegawa J, Nishino H. Triterpene acids from the leaves of Perilla frutescens and their anti-inflammatory and antitumor-promoting effects. Biosci Biotechnol Biochem. 2004;68:85-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 187] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 10. | Tokuda H, Ohigashi H, Koshimizu K, Ito Y. Inhibitory effects of ursolic and oleanolic acid on skin tumor promotion by 12-O-tetradecanoylphorbol-13-acetate. Cancer Lett. 1986;33:279-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 167] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 11. | Subbaramaiah K, Michaluart P, Sporn MB, Dannenberg AJ. Ursolic acid inhibits cyclooxygenase-2 transcription in human mammary epithelial cells. Cancer Res. 2000;60:2399-2404. [PubMed] |
| 12. | Mizushina Y, Iida A, Ohta K, Sugawara F, Sakaguchi K. Novel triterpenoids inhibit both DNA polymerase and DNA topoisomerase. Biochem J. 2000;350 Pt 3:757-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Shishodia S, Majumdar S, Banerjee S, Aggarwal BB. Ursolic acid inhibits nuclear factor-kappaB activation induced by carcinogenic agents through suppression of IkappaBalpha kinase and p65 phosphorylation: correlation with down-regulation of cyclooxygenase 2, matrix metalloproteinase 9, and cyclin D1. Cancer Res. 2003;63:4375-4383. [PubMed] |
| 14. | Liu J, Liu Y, Mao Q, Klaassen CD. The effects of 10 triterpenoid compounds on experimental liver injury in mice. Fundam Appl Toxicol. 1994;22:34-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 74] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Saraswat B, Visen PK, Agarwal DP. Ursolic acid isolated from Eucalyptus tereticornis protects against ethanol toxicity in isolated rat hepatocytes. Phytother Res. 2000;14:163-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 16. | Ovesna Z, Vachalková A, Horvathova K, Tothova D. Pentacyclic triterpenoic acids: new chemoprotective compounds. Minireview. Neoplasma. 2004;51:327-333. [PubMed] |
| 17. | Tian Z, Yang M, Huang F, Li K, Si J, Shi L, Chen S, Xiao P. Cytotoxicity of three cycloartane triterpenoids from Cimicifuga dahurica. Cancer Lett. 2005;226:65-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Miyase T, Shiokawa KI, Zhang DM, Ueno A. Araliasaponins I-XI, triterpene saponins from the roots of Aralia decaisneana. Phytochemistry. 1996;41:1411-1418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Sambrook J, Fritsch E F, Maniatis T. In Molecular cloning, A Laboratory Manual, 2nd ED. Cold Spring Harbor Laboratory press: New York 1989; . |
| 20. | Chen WF, Huang MH, Tzang CH, Yang M, Wong MS. Inhibitory actions of genistein in human breast cancer (MCF-7) cells. Biochim Biophys Acta. 2003;1638:187-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 81] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 21. | Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149116] [Cited by in RCA: 133709] [Article Influence: 5571.2] [Reference Citation Analysis (1)] |
| 22. | Setzer WN, Setzer MC. Plant-derived triterpenoids as potential antineoplastic agents. Mini Rev Med Chem. 2003;3:540-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 125] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 23. | Kujubu DA, Fletcher BS, Varnum BC, Lim RW, Herschman HR. TIS10, a phorbol ester tumor promoter-inducible mRNA from Swiss 3T3 cells, encodes a novel prostaglandin synthase/cyclooxygenase homologue. J Biol Chem. 1991;266:12866-12872. [PubMed] |
| 24. | Jones DA, Carlton DP, McIntyre TM, Zimmerman GA, Prescott SM. Molecular cloning of human prostaglandin endoperoxide synthase type II and demonstration of expression in response to cytokines. J Biol Chem. 1993;268:9049-9054. [PubMed] |
| 25. | DuBois RN, Awad J, Morrow J, Roberts LJ 2nd, Bishop PR. Regulation of eicosanoid production and mitogenesis in rat intestinal epithelial cells by transforming growth factor-alpha and phorbol ester. J Clin Invest. 1994;93:493-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 280] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 26. | Eberhart CE, Coffey RJ, Radhika A, Giardiello FM, Ferrenbach S, DuBois RN. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology. 1994;107:1183-1188. [PubMed] |
| 27. | Tucker ON, Dannenberg AJ, Yang EK, Zhang F, Teng L, Daly JM, Soslow RA, Masferrer JL, Woerner BM, Koki AT, Fahey TJ 3rd. Cyclooxygenase-2 expression is up-regulated in human pancreatic cancer. Cancer Res. 1999;59:987-990. [PubMed] |
| 28. | Buecher B, Broquet A, Bouancheau D, Heymann MF, Jany A, Denis MG, Bonnet C, Galmiche JP, Blottiere HM. Molecular mechanisms involved in the antiproliferative effect of two COX-2 inhibitors, nimesulide and NS-398, on colorectal cancer cell lines. Dig Liver Dis. 2003;35:557-565. [RCA] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Detjen KM, Welzel M, Wiedenmann B, Rosewicz S. Nonsteroidal anti-inflammatory drugs inhibit growth of human neuroendocrine tumor cells via G1 cell-cycle arrest. Int J Cancer. 2003;107:844-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Narayanan BA, Condon MS, Bosland MC, Narayanan NK, Reddy BS. Suppression of N-methyl-N-nitrosourea/testosterone-induced rat prostate cancer growth by celecoxib: effects on cyclooxygenase-2, cell cycle regulation, and apoptosis mechanism(s). Clin Cancer Res. 2003;9:3503-3513. [PubMed] |
| 31. | Kim DK, Baek JH, Kang CM, Yoo MA, Sung JW, Chung HY, Kim ND, Choi YH, Lee SH, Kim KW. Apoptotic activity of ursolic acid may correlate with the inhibition of initiation of DNA replication. Int J Cancer. 2000;87:629-636. [RCA] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 32. | Yamagishi N, Saito Y, Ishihara K, Hatayama T. Enhancement of oxidative stress-induced apoptosis by Hsp105alpha in mouse embryonal F9 cells. Eur J Biochem. 2002;269:4143-4151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 33. | Yamagishi N, Ishihara K, Saito Y, Hatayama T. Hsp105alpha enhances stress-induced apoptosis but not necrosis in mouse embryonal f9 cells. J Biochem. 2002;132:271-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 34. | Castelli C, Rivoltini L, Rini F, Belli F, Testori A, Maio M, Mazzaferro V, Coppa J, Srivastava PK, Parmiani G. Heat shock proteins: biological functions and clinical application as personalized vaccines for human cancer. Cancer Immunol Immunother. 2004;53:227-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 85] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 35. | Wadhwa R, Sugihara T, Yoshida A, Nomura H, Reddel RR, Simpson R, Maruta H, Kaul SC. Selective toxicity of MKT-077 to cancer cells is mediated by its binding to the hsp70 family protein mot-2 and reactivation of p53 function. Cancer Res. 2000;60:6818-6821. [PubMed] |