Published online Feb 7, 2006. doi: 10.3748/wjg.v12.i5.830
Revised: August 9, 2005
Accepted: August 25, 2005
Published online: February 7, 2006
- Citation: Mathonnet M, Descottes B, Valleix D, Labrousse F, Denizot Y. VEGF in hepatocellular carcinoma and surrounding cirrhotic liver tissues. World J Gastroenterol 2006; 12(5): 830-831
- URL: https://www.wjgnet.com/1007-9327/full/v12/i5/830.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i5.830
We read with a great interest the recent work of Deli and colleagues.[1] in the World Journal of Gastroenterology reporting vascular endothelial growth factor (VEGF) expression in hepatocellular carcinoma (HCC) and cirrhotic liver tissues. This well-documented work shows that VEGF was significantly higher in surrounding cirrhotic liver tissues than in HCC. Authors assessed VEGF expression using immunohistochemistry. The immunohistochemical staining is an efficient tool to assess the percentage of cells stained positively for VEGF but is not really efficient to estimate their true VEGF content. Evaluation of the VEGF protein by an enzyme-linked immunosorbent assay (ELISA) has been reported, by us and others[2-5], to be an efficient tool in order to assess tissue VEGF expression. We have, thus, tested whether the ELISA method might be an efficient tool in order to confirm data reporting higher amounts of VEGF in surrounding cirrhotic liver tissues than in HCC. Deli and colleagues.[1] also correctly pointed out that basic fibroblast growth factor (bFGF) has been reported to act cooperatively on VEGF expression[6]. We have, thus, also assessed bFGF tissue levels in order to search for a putative link between VEGF and bFGF levels in cirrhotic tissues.
The procedure of the present study followed the rules edited by the French National Ethics. Between January 1999 and December 2004, 12 consecutive (10 males and 2 females, mean age 67 ± 4 years, range 36-83 years) patients with an HCC developed in a cirrhosis underwent resection in the Department of Surgery of Limoges’ CHU. Specimens for the HCC tissue and the surrounding cirrhotic liver tissue were obtained during the surgical procedure and frozen at -80 °C until used. Cirrhosis was present in all cases with the cause being alcoholism or hepatitis C. Histologically, there was 5 well-differentiated HCC, 4 moderately differentiated HCC, and 3 poorly differentiated HCC. Tissue samples were homogenized in potassium phosphate buffer and VEGF and bFGF dosages were performed by specific ELISA assays (DuoSet®, R&D Systems Europe) according to the manufacturer's recommendations. Results in pg per mg of wet weight are reported as means ± SEM of 12 patients. Differences in mean content of VEGF and bFGF among tumour and the surrounding cirrhotic tissue were analysed by the Student's t-test for paired data. A P < 0.05 was considered statistically significant. Correlations between bFGF and VEGF values were assessed using linear regression analysis. A P < 0.05 and r > 0.5 were considered statistically significant.
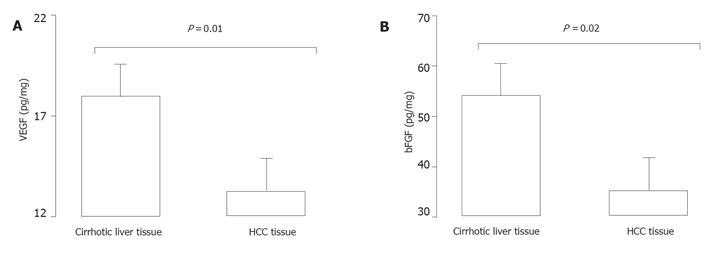
As shown in Figure 1A, VEGF contents were significantly elevated (P = 0.01) in the surrounding cirrhotic liver tissues (18.9 ± 2.1 pg/mg, range 2-33 pg/mg) than in HCC tissues (13.6 ± 1.9, range 2-25 pg/mg). As shown in Figure 1B, bFGF contents were significantly elevated (P = 0.02) in the surrounding cirrhotic liver tissues (55.4±6.0 pg/mg, range 15-94 pg/mg) than in HCC tissues (35.2 ± 8.5, range 4-63 pg/mg). While bFGF and VEGF levels were not correlated in HCC (r = 0.02, P = 0.66), bFGF and VEGF were strongly correlated in cirrhotic liver tissues (r = 0.85, P = 0.0005).
VEGF and bFGF amounts are elevated in cirrhotic liver tissue as compared with HCC ones. VEGF results obtained with the ELISA method corroborate those of Deli and colleagues.[1] concerning VEGF expression using immunohistochemistry. Immunohistochemistry and ELISA are, thus, two complementary methods that can be used in concert and bring concordant results. However, in our opinion, the main advantage of the ELISA method is the possibility to test several factors into the same tissue extract in order to highlight potential links between factors such as those found between VEGF and bFGF in the cirrhotic tissues (but not in HCC ones). As suggested by Deli and colleagues, VEGF (but also bFGF) may play an important role in the angiogenesis of liver cirrhosis. The higher expression of VEGF and bFGF suggested a possible role of angiogenesis in HCC carcinogenesis. Anti-angiogenic therapy potentially targeting VEGF and bFGF pathways could promise new strategies for the treatment of HCC.
S- Editor Guo SY L- Editor Elsevier HK E- Editor Cao L
| 1. | Deli G, Jin CH, Mu R, Yang S, Liang Y, Chen D, Makuuchi M. Immunohistochemical assessment of angiogenesis in hepatocellular carcinoma and surrounding cirrhotic liver tissues. World J Gastroenterol. 2005;11:960-963. [PubMed] |
| 2. | Landriscina M, Cassano A, Ratto C, Longo R, Ippoliti M, Palazzotti B, Crucitti F, Barone C. Quantitative analysis of basic fibroblast growth factor and vascular endothelial growth factor in human colorectal cancer. Br J Cancer. 1998;78:765-770. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 70] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Denizot Y, Descottes B, Truffinet V, Valleix D, Labrousse F, Mathonnet M. Platelet-activating factor and liver metastasis of colorectal cancer. Int J Cancer. 2005;113:503-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Denizot Y, Chianéa T, Labrousse F, Truffinet V, Delage M, Mathonnet M. Platelet-activating factor and human thyroid cancer. Eur J Endocrinol. 2005;153:31-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Tang TC, Poon RT, Lau CP, Xie D, Fan ST. Tumor cyclooxygenase-2 levels correlate with tumor invasiveness in human hepatocellular carcinoma. World J Gastroenterol. 2005;11:1896-1902. [PubMed] |
| 6. | Mandriota SJ, Pepper MS. Vascular endothelial growth factor-induced in vitro angiogenesis and plasminogen activator expression are dependent on endogenous basic fibroblast growth factor. J Cell Sci. 1997;110:2293-2302. [PubMed] |