Published online Feb 7, 2006. doi: 10.3748/wjg.v12.i5.697
Revised: June 28, 2005
Accepted: July 8, 2005
Published online: February 7, 2006
AIM: To determine the effects of the calcineurin inhibitors, cyclosporine and tacrolimus, on hepatitis C virus (HCV) replication and activity of recurrent hepatitis C in patients post liver transplantation.
METHODS: The data of a cohort of 107 patients who received liver transplantation for HCV-associated liver cirrhosis between 1999 and 2003 in our center were retrospectively analyzed. The level of serum HCV-RNA and the activity of recurrent hepatitis were compared between 47 patients who received either cyclosporine or tacrolimus as the primary immunosuppressive agent and an otherwise similar immunosuppressive regimen which did not lead to biliary complications within the first 12 mo after transplantation.
RESULTS: HCV-RNA increased within 3 mo after transplantation but the differences between the cyclosporine group and the tacrolimus group were insignificant (P = 0.49 at 12 mo). In addition, recurrent hepatitis as determined by serum transaminases and histological grading of portal inflammation and fibrosis showed no significant difference after 12 mo (P = 0.34).
CONCLUSION: Cyclosporine or tacrolimus as a primary immunosuppressive agent does not influence the induction or severity of recurrent hepatitis in HCV-infected patients after liver transplantation.
-
Citation: Hilgard P, Kahraman A, Lehmann N, Seltmann C, Beckebaum S, Ross RS, Baba HA, Malago M, Broelsch CE, Gerken G. Cyclosporine
versus tacrolimus in patients with HCV infection after liver transplantation: Effects on virus replication and recurrent hepatitis. World J Gastroenterol 2006; 12(5): 697-702 - URL: https://www.wjgnet.com/1007-9327/full/v12/i5/697.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i5.697
Hepatitis C virus (HCV) is a leading cause of chronic hepatitis with about 170-190 million people infected worldwide[1,2]. The treatment of choice for patients with end-stage liver disease associated with HCV infection is liver transplantation (LTx) and in fact, HCV is the main indication for liver transplantation among adults in Europe and USA, accounting for approximately 50% of cases[3]. Since HCV persists not only in hepatocytes but also in leukocytes, lymph nodes and most likely additional tissues[4-6], the virus is redistributed into the donor liver after LTx almost immediately in virtually all patients[7,8]. However, the severity and clinical course of the resulting reinfection hepatitis vary widely, ranging from deleterious fibrosing cholestatic hepatitis with failure of the allograft within 1 year to a rather mild hepatitis with progression to cirrhosis within 5 years in 20-30% of cases[9,10].
A main factor determining the severity of recurrent hepatitis C after transplantation may be immuno-suppression[11]. Of particular concern is the fact that the prognosis of recurrent HCV infection seems to deteriorate in recent years, which has been attributed to the use of donor organs of less optimal quality as well as changes in the immunosuppressive therapy such as steroid-free regimens or the combination of particular drugs[12,7]. Considering that retransplantation remains the only treatment for patients with graft failure due to HCV recurrence, optimization of the immunosuppressive regimens might be a key aspect to improve the prognosis of chronic hepatitis C after transplantation.
The two most frequently used basic immuno-suppresive drugs are cyclosporine A (CsA) and tacrolimus (Tac). Although CsA and Tac are structurally different, they share a similar mode of immunosuppressive action by inhibition of the transcription factor NFAT and consecutive reduction of inflammatory gene expression in activated T cells[13-15]. The main mechanism by which immunosuppressive therapy with calcineurin inhibitors accelerates the clinical course of post-transplantation hepatitis C is their influence on the replication of the virus. Immunosuppressive therapy after LTx of patients with chronic HCV infection is associated with significantly increased serum viral load compared to the values of the same patients before transplantation[7,11], suggesting decreased systemic immune responses against the virus. Locally in the liver, the uncontrolled replication of the virus may cause an enhanced immune response, resulting in accelerated hepatocellular damage and fibrosis.
Whether a particular calcineurin inhibitor has an advantage in immunosuppressive therapy of HCV-infected patients post LTx is still highly controversial[16-19]. To further clarify this issue, the aim of this study was to determine the effects of CsA and Tac on serum HCV-RNA level as well as on the inflammatory activity of recurrent hepatitis in patients with chronic HCV infection after LTx.
The data of 107 consecutive patients within the first year after liver transplantation due to hepatitis C-associated liver cirrhosis were retrospectively reviewed. Patients underwent LTx between June 1998 and December 2003 at our center either as cadaveric-(orthotopic) or as living donor-related liver transplantation. In order to compensate for missing randomization in this retrospective study, only 47 patients who received either cyclosporine or tacrolimus as the primary immunosuppressive agent and an otherwise similar immunosuppressive regimen which did not lead to acute or chronic rejection and biliary complications within the first 12 mo after transplantation, forming a relatively homogenous subgroup, were included in this study. A further exclusion criterion was early death after transplantation. During the first year all patients underwent routine liver biopsy 3-5 mo after LTx, independent from serum liver enzyme activities. Every rise of transaminases or cholestasis parameters was followed by additional liver biopsies to differentiate between acute or chronic rejection, cholestasis and hepatitis C reinfection. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Local Ethics Committee.
The basic immunosuppressive agent used in all the patients was either CsA or Tac. The dose of the drugs was controlled in light of the serum level, which was adjusted between 140 and 180 ng/mL for CsA, and 10 and 15 ng/mL for Tac within the first 3 mo. After 3 mo, the serum level of CsA and Tac was reduced to 100-120 ng/mL and 7-10 ng/mL, respectively. During the first 3 mo. after LTx, CsA, and Tac were routinely combined with corticosteroids (prednisolone) at an initial dose of 1 mg/kg body weight and reduced to 5 mg daily within 4 weeks. After 3 mo steroids were discontinued. In addition, all the patients included in this study received 1 000-2 000 mg mycophenolate mofetil (MMF), per day for the first 12 mo post LTx.
HCV infection status was evaluated by the examination of the patients’ sera for the presence of anti-HCV antibodies and HCV-RNA. Anti-HCV antibodies were detected by a commercially available enzyme immunoassay (Monolisa anti-HCV plus, Biorad, Munich) according to the manufacturer’s instructions. Qualitative detection of serum HCV-RNA was performed by a RT-PCR-based (Cobas Amplicor HCV 2.0 assay, Roche Diagnostics, Mannheim, Germany) assay or a transcription-mediated amplification (TMA)-based assay (VERSANT HCV-RNA qualitative, Bayer Diagnostics, Fernwald, Germany). HCV-RNA concentrations were determined by commercially available second and third generation branched DNA (bDNA) assays (VERSANT HCV-RNA 2.0 and 3.0, Bayer Diagnostics, Fernwald, Germany). To obtain HCV-RNA titers in IU/mL, the bDNA 2.0 assay was calibrated against the WHO HCV Standard 96/790[20], as described in full details elsewhere[21,22]. Values below the lower limit of sensitivity of 615 IU/mL were set to 615 IU/mL divided by 2.
Inflammatory activity in the graft due to hepatitis induced by HCV reinfection was in all patients under surveillance determined by routine measurement of transaminases (ALT or GPT and AST or GOT) as well as alkaline phosphatase and gamma-glutamyl-transferase and bilirubin (every 7-30 d, interval depending on the time after LTx and level of elevation). Every unexplained rise was immediately followed by B-mode and duplex-/Doppler ultrasonographic analysis of liver parenchyma and the supplying vessels. If thrombosis of the hepatic artery and congestion of the biliary system were excluded, liver biopsy was performed. In analogy to patients pre LTx, recurrent hepatitis C severity was routinely determined by the modified Knodell hepatic activity index (HAI) with subscores for piecemeal necrosis (0-4), confluent necrosis (0-6), lytic necrosis/apoptosis/focal inflammation (0-4) and portal inflammation (0-4), as well as fibrosis staging (0-6)[23].
Virological, biochemical, and histological data were collected in a computer database (Microsoft Excel). Statistical analyses were performed utilizing SAS System software, release 8.2 (SAS Institute Inc., Cary, NC, USA). Qualitative characteristics were analyzed by χ2 test or Fisher’s exact test as appropriate. Two-sided P-values and changes in HCV-RNA concentrations and ALT activities were evaluated by Mann-Whitney U-test. Correlation coefficients were reported as rank (Spearman) correlations. P < 0.05 was considered statistically significant. In this explorative analysis, no adjustment for multiple testing was performed.
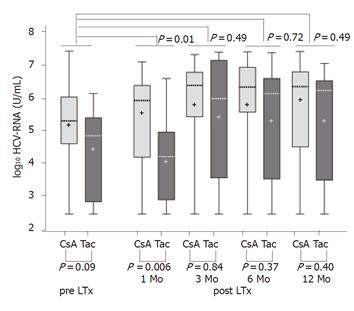
Of the 47 patients included in this study, 35 received CsA and 12 received Tac as the primary immunosuppressive agent. The baseline characteristics of the two patient groups showed no statistically significant differences (Table 1). Patients underwent liver transplantation at least 12 mo before the study. In both groups of patients, serum HCV-RNA level was quantitatively assessed at 1, 3, 6, and 12 mo after liver transplantation and compared to the HCV-RNA level prior to the operation. Figure 1 depicts these data on logarithmic scale (log10) as a box-whisker’s plot of HCV-RNA in relation to the primary immunosuppressive drug (CsA or Tac) and the time after LTx. P-values (Mann-Whitney U-test) at the top of the Figure express the group differences of changes in HCV serum RNA level (virus load), after 1, 3, 6, and 12 mo and the last measurement pre transplantation as indicated. P-values at the bottom of the Figure are the group differences between HCV-RNA concentrations at the given time of measurement. Boxes indicate the lower and upper quartiles and median, ‘+’ indicates mean.
| CsA | Tac | P-value | |
| Number of patients | 35 | 12 | |
| Age | 28-64 | 27-61 | 0,20 |
| Gender | F = 9, m = 26 | F = 3, m = 9 | 0.96 |
| OLT | 31 | 10 | |
| LDLT | 4 | 2 | 0.64 |
| HCV genotype | gt1 = 32/35, | gt1 = 10/12, | 0.46 |
| gt2 = 1/35, | gt2 = 0/12, | ||
| gt3 = 2/35 | gt3 = 2/12 |
Serum HCV-RNA concentrations showed a substantial rise (between 10- and 1 000-fold) after LTx. The elevation of HCV-RNA levels developed between 1 and 3 mo post LTx, at which point the elevation was the most pronounced and continued then throughout the entire observation period of 12 mo. The patients who received tacrolimus for immunosuppression had a lower mean HCV-RNA level pre LTx than those who received CsA, but the difference was not statistically significant (P = 0.09). One month post LTx this group difference in the HCV-RNA levels was still present and even slightly accentuated, reaching statistical significance at this point (P = 0.006). However, 3 mo post LTx the difference was significantly diminished neither at this time (P = 0.84) nor at 6 (P = 0.37) or 12 mo (P = 0.40) post LTx. The group difference reached statistical significance.
A statistical significance between the levels of HCV-RNA in CsA and Tac groups was evaluated by comparison of the group difference at each given time point after LTx with the group difference before LTx. This analysis revealed no relevant dissimilarity between serum HCV-RNA concentrations in patients who received CsA or Tac for immunosuppression. A slightly higher rise in the tacrolimus group particularly 3 mo post LTx was statistically insignificant (P = 0.49). The differences at 6 and 12 mo between both the groups were also not significant (P = 0.72, P = 0.49). Table 2 lists the net group differences of the changes on a log10-scale in serum HCV-RNA between the pre-LTx and post LTx values.
| Mo | Mean | 95%CI | P-value1 |
| 1 | 0.40 | (–0.28; 1.08) | 0.11 |
| 3 | –0.50 | (–1.46; 0.47) | 0.49 |
| 6 | –0.43 | (–1.50; 0.64) | 0.72 |
| 12 | –0.56 | (–1.64; 0.52) | 0.49 |
A power analysis revealed that with the given patient number, the group differences of the changes in serum HCV-RNA concentrations should be on the order 10-fold higher in order to be detectable with a power of 80%. In fact, the strongest deviation from the null hypothesis of no group difference of changes was not a significantly (P = 0.11) stronger increase by a factor of 2.5 from pre LTx to the 1 mo measurement in the CsA as compared to the tacrolimus group, opposing a possible superiority of CsA.
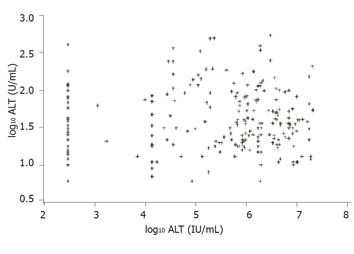
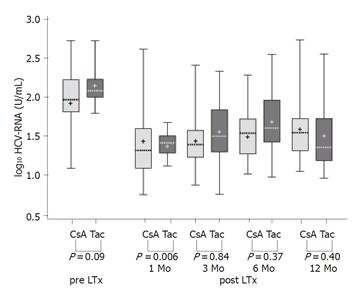
The value of HCV serum RNA level in predicting the inflammatory activity and progression of fibrosis was analyzed by a scatter plot of serum ALT, mirroring the activity of recurrent hepatitis, in dependence of serum HCV-RNA levels (Figure 2). The correlation coefficient was almost equal to zero (r = 0.01, P = 0.92), suggesting no significant association between levels of HCV-RNA and intrahepatic inflammatory activity. Therefore HCV-RNA levels post transplantation may not be a valid predictor of allograft inflammation. Differences in the progression of recurrent hepatitis were examined by serum transaminase level and histology in the two patient groups. Figure 3 shows the ALT levels (logarithmically scaled) as a box-whisker’s plot of enzyme activity in correlation to immunosuppression (CsA group or FK 506 group) and the time after LTx. P-values (Mann-Whitney U-test) at the bottom of the Figure are for group differences between ALT activity at the time of measurement. Boxes indicate the lower and upper quartiles and median, ‘+’ indicates mean.
In comparison to the level before LTx, the ALT level fell significantly immediately after the operation, with the lowest values 1 mo post LTx. After 3 mo especially after 6 mo ALT levels rose again and stayed elevated over the entire observation period of 12 mo, indicating the development of recurrent chronic hepatitis C. The ALT activity after 3 and 6 mo was increased in the CsA group. but the difference was not statistically significant (3 mo: P = 0.36, 6 mo: P = 0.65). Analysis of AST activity revealed similar kinetics post LTx with lower absolute values and also no statistically significant group differences (data not shown).
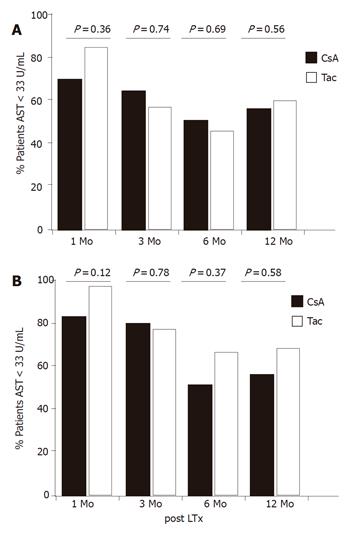
In conjunction with these analyses, examination of the proportion of patients with normal activities of transaminases post LTx revealed that 70% of patients in the CsA group and 83% in the Tac group had normal ALT activities 1 mo post LTx (Figure 4A). Recurrent hepatitis led to a significant decrease of the proportion of patients with normal ALT values after 3 and 6 mo (48% in the CsA group and 42% in the Tac group). While the proportion of patients with normal ALT at these time points was slightly higher in the CsA group than in the Tac group which was reversed after 12 mo, only 49% of patients in the CsA group but 58% of patients in the Tac group had normal ALT values. However, the group differences were statistically significant neither at 6 mo (P = 0.69) nor at 12 mo (P = 0.56). The proportion of patients with normal AST changed similarly (Figure 4B), indicating no advantage for CsA in suppressing inflammatory activity.
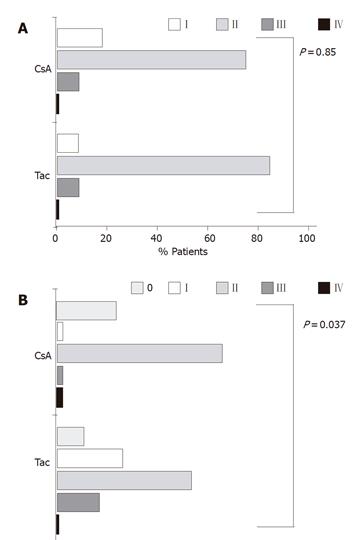
To further evaluate the potential differences in the activation and maintenance of recurrent hepatitis C between the two groups, we compared necroinflammatory activity and grade of fibrosis according to HAI scores in the liver histology taken 3-5 mo post LTx. The majority of patients in both groups had a grade II inflammation (74% of patients in the CsA group and 83% of patients in the Tac group), indicating that recurrent hepatitis occurred in a significant proportion of patients in the absence of elevated serum transaminases (Figure 5A). The total group differences including all inflammation grades between the CsA and Tac groups were statistically negligible (P = 0.85). Examination of fibrosis revealed a larger proportion of patients in the CsA group than in the Tac group scored with grade 0 (26% vs 8.3%, Figure 5B). However, at grade I this relation was reversed (2.9% vs 25 %). In addition, more patients in the CsA group were scored with grade II fibrosis, while patients in the Tac group were more frequently scored with grade III fibrosis. The number of patients presenting with grade IV fibrosis was very small in both groups. Due to the changes in grades 0 and I, these overall group differences were statistically significant (P = 0.03). However, if grades 0 and I were analyzed as one group, no statistical differences were found (P = 0.34).
The marked increase in serum HCV-RNA levels after LTx is closely associated with the immunosuppressive therapy[7,11,24]. Our study also confirmed that serum HCV-RNA levels increased in the early (3 mo) post-transplant period, when immunosuppression was the highest. Although there is evidence that immunosuppression accelerates the progression of chronic HCV infection to cirrhosis, no clear association has been found between the type or dose of immunosuppressive regimen and the outcome of post-transplant HCV recurrence. CsA and Tac are the most common immunosuppressive drugs used after LTx, but their exact impact on the incidence, severity, time of onset and outcome of HCV recurrence is unclear[10]. In this context, Watashi et al [17] showed that CsA but not Tac can inhibit HCV replication in vitro, which raises the question whether HCV-infected patients treated with CsA have an advantage with regard to the progression of recurrent hepatitis and therefore graft survival as compared to those treated with Tac.
Our results did not confirm the function of CsA in inhibiting HCV replication as compared to Tac. Neither the differences at any time of examination nor the differences in serum HCV-RNA levels after LTx were in favor of CsA. In addition, there were no significant differences in inflammation of the allograft between the CsA and Tac groups. as determined by serum transaminase activities and histological grading of intrahepatic necroinflammatory activity. The slight differences in the grade of fibrosis, suggesting a lower grade of fibrosis in the CsA group were not statistically significant. While a study showed that HCV-infected patients treated with Tac have an improved survival in comparison to those treated with CsA[16], our results are in accordance with a recently published study, which also found no advantage for either immunosuppressive agent[19]. However, Watashi et al [17] showed that CsA can inhibit HCV replication in hepatocytes. In the last several years. a number of cell culture propagation systems based on the infection of primary human[25,26] or chimpanzee[27] hepatocytes or hepatic cell lines[28,29] have been reported. There is a general agreement that all these systems have a poor validity and reproducibility. Viral proteins and particles have never been convincingly detected and viral RNA can be quantified only by the sensitive PCR methodology[30,31]. In HCV research, these limitations of the infection-based cell culture systems have led to the development of a selectable HCV replicon system[32]. In this system, a subgenomic part of HCV-RNA can replicate in a human hepatoma cell line (HuH-7) to unphysiologically high levels, suggesting that this system is highly artificial and may not adequately mirror the situation in vivo. An additional important aspect regarding both cell culture systems is the absence of the immune system. Even if CsA has a HCV suppressing effect in vivo, this effect is by far overweighed by the immunosuppressive effects of the drug, resulting in a decreased control of HCV replication by the immune system.
In conclusion, our study does not justify a recommendation for CsA as a primary immunosuppressive agent for the treatment of HCV-infected patients post LTx. Further prospective studies should be conducted to clarify this issue. To vigorously improve the graft survival and prognosis of HCV-infected patients post LTx, development of new therapeutic agents directly modifying HCV-replication and/or inflammatory activity of recurrent hepatitis C in combination with alternative immunosuppressive regimens remains a high priority goal.
S- Editor Wang XL and Guo SY L- Editor Elsevier HK E- Editor Cao L
| 1. | Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med. 2001;345:41-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2042] [Cited by in RCA: 2025] [Article Influence: 84.4] [Reference Citation Analysis (0)] |
| 2. | Alter MJ, Kruszon-Moran D, Nainan OV, McQuillan GM, Gao F, Moyer LA, Kaslow RA, Margolis HS. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med. 1999;341:556-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1934] [Cited by in RCA: 1900] [Article Influence: 73.1] [Reference Citation Analysis (0)] |
| 3. | Curry MP. Hepatitis B and hepatitis C viruses in liver transplantation. Transplantation. 2004;78:955-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Vargas HE, Laskus T, Radkowski M, Wilkinson J, Balan V, Douglas DD, Harrison ME, Mulligan DC, Olden K, Adair D. Detection of hepatitis C virus sequences in brain tissue obtained in recurrent hepatitis C after liver transplantation. Liver Transpl. 2002;8:1014-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Yan FM, Chen AS, Hao F, Zhao XP, Gu CH, Zhao LB, Yang DL, Hao LJ. Hepatitis C virus may infect extrahepatic tissues in patients with hepatitis C. World J Gastroenterol. 2000;6:805-811. [PubMed] |
| 6. | Lamelin JP, Zoulim F, Trépo C. Lymphotropism of hepatitis B and C viruses: an update and a newcomer. Int J Clin Lab Res. 1995;25:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Gruener NH, Jung MC, Schirren CA. Recurrent hepatitis C virus infection after liver transplantation: natural course, therapeutic approach and possible mechanisms of viral control. J Antimicrob Chemother. 2004;54:17-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Porter SB, Reddy KR. Factors that influence the severity of recurrent hepatitis C virus following liver transplantation. Clin Liver Dis. 2003;7:603-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Prieto M, Berenguer M, Rayón JM, Córdoba J, Argüello L, Carrasco D, García-Herola A, Olaso V, De Juan M, Gobernado M. High incidence of allograft cirrhosis in hepatitis C virus genotype 1b infection following transplantation: relationship with rejection episodes. Hepatology. 1999;29:250-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 450] [Cited by in RCA: 414] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 10. | Forman LM, Lewis JD, Berlin JA, Feldman HI, Lucey MR. The association between hepatitis C infection and survival after orthotopic liver transplantation. Gastroenterology. 2002;122:889-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 888] [Cited by in RCA: 829] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 11. | Charlton M. Liver biopsy, viral kinetics, and the impact of viremia on severity of hepatitis C virus recurrence. Liver Transpl. 2003;9:S58-S62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 46] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Berenguer M, Ferrell L, Watson J, Prieto M, Kim M, Rayon M, Cordoba J, Herola A, Ascher N, Mir J. HCV-related fibrosis progression following liver transplantation: increase in recent years. J Hepatol. 2000;32:673-684. |
| 13. | Lorber MI. The mechanism of ciclosporin immunosuppression. Year Immunol. 1989;4:253-263. [PubMed] |
| 14. | Cristillo AD, Macri MJ, Bierer BE. Differential chemokine expression profiles in human peripheral blood T lymphocytes: dependence on T-cell coreceptor and calcineurin signaling. Blood. 2003;101:216-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Yin M, Ochs RS. Mechanism for the paradoxical inhibition and stimulation of calcineurin by the immunosuppresive drug tacrolimus (FK506). Arch Biochem Biophys. 2003;419:207-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Wiesner RH. A long-term comparison of tacrolimus (FK506) versus cyclosporine in liver transplantation: a report of the United States FK506 Study Group. Transplantation. 1998;66:493-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 156] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 17. | Watashi K, Hijikata M, Hosaka M, Yamaji M, Shimotohno K. Cyclosporin A suppresses replication of hepatitis C virus genome in cultured hepatocytes. Hepatology. 2003;38:1282-1288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 425] [Cited by in RCA: 409] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 18. | Shiffman ML, Vargas HE, Everson GT. Controversies in the management of hepatitis C virus infection after liver transplantation. Liver Transpl. 2003;9:1129-1144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Martin P, Busuttil RW, Goldstein RM, Crippin JS, Klintmalm GB, Fitzsimmons WE, Uleman C. Impact of tacrolimus versus cyclosporine in hepatitis C virus-infected liver transplant recipients on recurrent hepatitis: a prospective, randomized trial. Liver Transpl. 2004;10:1258-1262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 103] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 20. | Saldanha J, Lelie N, Heath A. Establishment of the first international standard for nucleic acid amplification technology (NAT) assays for HCV RNA. WHO Collaborative Study Group. Vox Sang. 1999;76:149-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 193] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 21. | Jorgensen PA, Neuwald PD. Standardized hepatitis C virus RNA panels for nucleic acid testing assays. J Clin Virol. 2001;20:35-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Ross RS, Viazov S, Sarr S, Hoffmann S, Kramer A, Roggendorf M. Quantitation of hepatitis C virus RNA by third generation branched DNA-based signal amplification assay. J Virol Methods. 2002;101:159-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 23. | Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween RN. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3521] [Cited by in RCA: 3782] [Article Influence: 126.1] [Reference Citation Analysis (1)] |
| 24. | Garcia-Retortillo M, Forns X, Feliu A, Moitinho E, Costa J, Navasa M, Rimola A, Rodes J. Hepatitis C virus kinetics during and immediately after liver transplantation. Hepatology. 2002;35:680-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 422] [Cited by in RCA: 381] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 25. | Fournier C, Sureau C, Coste J, Ducos J, Pageaux G, Larrey D, Domergue J, Maurel P. In vitro infection of adult normal human hepatocytes in primary culture by hepatitis C virus. J Gen Virol. 1998;79:2367-2374. [PubMed] |
| 26. | Rumin S, Berthillon P, Tanaka E, Kiyosawa K, Trabaud MA, Bizollon T, Gouillat C, Gripon P, Guguen-Guillouzo C, Inchauspé G. Dynamic analysis of hepatitis C virus replication and quasispecies selection in long-term cultures of adult human hepatocytes infected in vitro. J Gen Virol. 1999;80:3007-3018. [PubMed] |
| 27. | Lanford RE, Sureau C, Jacob JR, White R, Fuerst TR. Demonstration of in vitro infection of chimpanzee hepatocytes with hepatitis C virus using strand-specific RT/PCR. Virology. 1994;202:606-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 226] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 28. | Seipp S, Mueller HM, Pfaff E, Stremmel W, Theilmann L, Goeser T. Establishment of persistent hepatitis C virus infection and replication in vitro. J Gen Virol. 1997;78:2467-2476. [PubMed] |
| 29. | Tagawa M, Kato N, Yokosuka O, Ishikawa T, Ohto M, Omata M. Infection of human hepatocyte cell lines with hepatitis C virus in vitro. J Gastroenterol Hepatol. 1995;10:523-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 30. | Pietschmann T, Bartenschlager R. Tissue culture and animal models for hepatitis C virus. Clin Liver Dis. 2003;7:23-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 31. | Grakoui A, Hanson HL, Rice CM. Bad time for Bonzo Experimental models of hepatitis C virus infection, replication, and pathogenesis. Hepatology. 2001;33:489-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Lohmann V, Körner F, Koch J, Herian U, Theilmann L, Bartenschlager R. Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science. 1999;285:110-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2294] [Cited by in RCA: 2251] [Article Influence: 86.6] [Reference Citation Analysis (0)] |