Published online Dec 14, 2006. doi: 10.3748/wjg.v12.i46.7527
Revised: October 15, 2006
Accepted: October 23, 2006
Published online: December 14, 2006
AIM: To study the protective effect of a natural antioxidant, melatonin, against multistress condition induced lipid peroxidation via determination of gastric damage and plasma malondialdehyde (MDA) level by high performance liquid chromatography in rats.
METHODS: We compared indomethacin-induced gastric damage and MDA plasma level in three groups of rats: unoperated, bile duct ligated and sham-operated and evaluated the role of the melatonin on gastric damage and plasma MDA level. Indomethacin and melatonin were injected intraperitoneally in doses of 50 mg/kg and 20 mg/kg, respectively. Animals were killed 4 h after indomethacin injection.
RESULTS: Indomethacin induced more severe gastric damage and plasma MDA level in bile duct ligated animals was significantly higher (3.1 ± 0.04 μmol/L) than sham (2.8 ± 0.04 μmol/L) and unoperated animals (1.4 ± 0.08 μmol/L). Pretreatment with melatonin reduced indomethacin-induced gastric damage and plasma MDA level.
CONCLUSION: Considering the results of this study, we suggest that in multistress conditions the intensity of gastric damage and the plasma MDA level are great and melatonin reduces the negative effect of lipid peroxidation and cell damage by oxidative stress in multistress conditions due to its antioxidizing activity.
-
Citation: Kiarostami V, Samini L, Ghazi-Khansari M. Protective effect of melatonin against multistress condition induced lipid peroxidation
via measurement of gastric mucosal lesion and plasma malondialdehyde levels in rats. World J Gastroenterol 2006; 12(46): 7527-7531 - URL: https://www.wjgnet.com/1007-9327/full/v12/i46/7527.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i46.7527
It has been accepted that the pathogenesis of ulcer is complex and related to different pathogenic factors. The most common side-effect of indomethacin (NSAID) is gastric mucosal damage. The inhibition of the biosynthesis of gastric prostaglandins is accepted as a main mechanism implicated in NSAID-induced gastropathy[1,2]. Oxidative stress is involved in this pathology and it has been suggested that active oxygen metabolites play an important role in gastric damage induced by aspirin[3]. The focal ischemic areas produced as a result of inhibition of prostaglandin synthesis, may eventually produce tissue-damaging mediators such as oxygen-derived free radicals[1]. The break down of lipid peroxide in the biological system produces a number of aldehydes such as malondialdehyde (MDA), which are cytotoxic by reacting with lipids, protein and nucleic acids[4]. It has been shown that gastric mucosa of cholestatic rats is more vulnerable than that of normal animals to NSAIDs and this has been referred to different factors such as increased gastric acid secretion, decreased prostaglandins and increased free radical formation and accumulation of endogenous opioids[5].
Melatonin, a pineal secretory product, was recently found to be a potent free radical scavenger and antioxidant, especially hydroxyl radical (•OH) and peroxyl radical. In in vitro and in vivo experiments melatonin in different models of oxidative stress has been found to protect tissues against oxidant damage induced by various free radical generating agents[6-9]. It has been shown that melatonin exerts protective and therapeutic effects against cholestatic liver injury and its associated oxidative stress in rats subjected to BDL through its direct and indirect antioxidant actions as well as its anti-inflammatory effects[10-14].
The aim of this study was to investigate the antioxidant effect of melatonin on multistress conditions via assessment of gastric lesion and determination of MDA concentration in plasma as a lipid peroxidation biomarker. We compared gastric damage and plasma MDA level in 3 groups of rats: unoperated (UNOP), bile duct ligated (BDL) and sham-operated (SHAM) and evaluated the anti-oxidative effect of melatonin in these groups.
The following chemicals were used: 1,1,3,3- tetramethoxy-propane (Sigma), butylated hydroxytoluene (Sigma), 2-thiobarbituric acid (Serva), high performance liquid chromatography (HPLC) grade methanol and ethanol (Merck), melatonin (Nature’s Bounity), ketamin HCl (Rotex Medica) and Promethazine HCl (Sigma).
Chemical solutions were prepared using distilled deionized water. Butylated hydroxytoluene (BHT) solution was prepared in ethanol to a final concentration of 0.05% BHT. An 0.44 mol/L phosphoric acid solution was obtained by diluting 1 mL concentrated phosphoric acid to 100 mL final volume. 2-thiobarbituric acid (TBA) was dissolved in water on a stirring hot-plate at 50-55°C to a concentration of 42 mmol/L.
Male Sprague-Dawley rats weighing 200-250 mg were used in this experiment. All animals were given free access to food and water. The animals were handled in accordance with the criteria and recommendations of the ethics committee on animal experiments of the Medical School Tehran University of Medical Sciences. Animals were divided into three groups.
First group (UNOP, n = 5), second group (BDL, n = 10), third group (SHAM, n = 10). Animals in BDL and SHAM groups were divided randomly into sham plus saline plus indomethacin, bile duct ligation plus saline plus indomethacin, sham plus melatonin plus indomethacin and bile duct ligation plus indomethacin plus indomethacin subgroups, respectively; each subgroup consisted of 5 rats.
Laparotomy was performed under general anesthesia induced by intraperitoneal (IP) injection of ketamin (60 mg/kg) and chlorpromazine (20 mg/kg) in BDL rats, the common bile duct was isolated and doubly ligated. In SHAM rats the bile duct was identified, manipulated and left in situ. Then the abdominal wall was closed in two layers[15]. Five days after surgery animals were fasted but with access to water and during this period were housed in individual cages with a wire-mesh floor to prevent coprophagy. Experiments were performed 7 d after surgery, when the BDL group had shown overt jaundice. Rats were divided into 5 groups.
UNOP group: which were injected with saline at 0 and 30 min and were killed under ether anesthesia 4 h after last injection and 2 mL blood were drawn from the heart ventricle into a syringe containing EDTA (ethylenediaminetetraacetic acid) with a final EDTA concentration 1 mg/mL and transferred to plastic tubes and centrifuged for 10 min at 4000 rpm at room temperature. The aliquots were kept frozen at -80°C until analysis. Plasma samples in this temperature are stable for at least one year[16]. Then the stomachs were immediately removed and cut along the greater curvature and the stomach contents were washed out with saline and gastric lesions were fixed with 20% formaldehyde for 5 min.
BDL group: which were injected with saline (1 mL/kg, ip) and 30 min later treated with indomethacin (50 mg/kg, ip) and were killed under ether anesthesia 4 h after last injection. Plasma samples and stomachs were prepared like the UNOP group.
BDL group: which were pretreated with melatonin (20 mg/kg, ip)[17] and 30 min later treated with indomethacin (50 mg/kg, ip) and were killed under ether anesthesia 4 h after last injection. Plasma samples and stomachs were prepared like the UNOP group.
SHAM group: which were injected with saline (1 mL/kg, ip) and 30 min later treated indomethacin (50 mg/kg, ip) and were killed under ether anesthesia 4 h after last injection. Plasma samples and stomachs were prepared like the UNOP group.
SHAM group: which were pretreated with melatonin (20 mg/kg, ip) and 30 min later treated with indomethacin (50 mg/kg, ip) and were killed under ether anesthesia 4 h after last injection. Plasma samples and stomachs were prepared like the UNOP group.
Gastric lesions were assessed macroscopically and evaluated according to J-score[18]. The J-score was calculated by classifying the lesions by diameter; 0-1 mm = 1; 1-2 mm = 2; greater than 2 mm = 3. The score was defined as the sum of these points in each rat[19]. Plasma analysis was performed immediately after thawing the plasma samples and lipid peroxidation was monitored by MDA measurement by HPLC-based assay of MDA-TBA adduct.
Standards and control samples were prepared using TMP with the stock standard solution containing 100 μmol/L TMP in a 40% ethanol solution. Standards were prepared through serial dilution of stock standards with ethanol solution to obtain concentrations of 4, 3, 2, 1, 0.5 and 0 (blank) μmol/L TMP. The concentration of TMP solution did not change after three months at 4°C (refrigerator)[7]. The standard curve was prepared freshly for analysis each day as well as control samples of 4, 3, 2, 1, 0.5 and 0 (blank) μmol/L TP.
Sample derivatization was carried out in 2 mL capacity plastic centrifuge tubes fitted with screw-on caps. To a 150 μL aliquot of sample or TMP standard, 50 μL BHT, 400 μL H3PO4 and 100 μL TBA solution were added. Sample tubes were capped tightly, vortexed, and then heated for 1 h in a 100°C water bath. Following heat derivatization, samples were placed on an ice-water (0°C) water bath for 5 min to cool, with 250 μL n-butanol subsequently added to each tube for extraction of the MDA-TBA complex. Tubes were vortexed 5 min and then centrifuged 3 min at 14 000 rpm to separate the two phases[20]. Aliquots of 20 μL removed from n-butanol layers of each sample and injected in HPLC for analysis without evaporation[20]. Since MDA degrade approximately 10% per hour, the best performance of assay requires analyzing samples within an hour of derivatization[20].
Chromatographic determination was performed on a Cecil 1100 series high performance- liquid chromatograph equipped with an 1100 series pump and a fluorescence detector which was set at an excitation wavelength of 515 nm and emission wavelength of 553 nm. A HP 3395 integrator was employed to record retention times, chromatograms and evaluate peak areas. The column was a Pefectsil Target (ODS, 150 mm × 4.6 mm, 3-5 μ particles). Elution was performed isocraticaly with a mixture of methanol-buffer (40:60 v/v) at a flow rate of 1.0 mL/min at room temperature. The buffer was 50 mmol/L potassium monobasic phosphate (anhydrous) with an adjusted pH of 6.8 using a 5 mmol/L potassium hydroxide solution. In this study, we used the Agarwal method for determination of MDA[20].
Calibration curves were created for 6 different concentrations (0-4 μmol/L) by plotting the peak areas versus the nominal MDA concentrations. There was a significant linear relationship between MDA concentration in water and plasma and peak area obtained by fluorescence (water: Y = 1 642 598X + 484 484; r2 = 0.999; plasma: Y = 1 888 481X + 2 706 994; r2 = 0.999). Furthermore the slopes of peak areas to MDA levels were parallel in plasma and water, and that no matrix effect existed when n-butanol was added as an extracting solvent, suggesting that n-butanol is superior as an extracting solvent.
The accuracy was determined by evaluation of analytical recovery after addition of known amounts of standard solution to plasma. After homogenizing the samples, the MDA-TBA adduct was measured as described in the sample preparation section. The limit of detection was computed by standard method[21]. The average of recovery of MDA-TBA adducts was 99%.
Data were analyzed statistically by one-way analysis of variance (ANOVA) and expressed as mean ± SD. The P values less than 0.05 were considered statistically significant. If a significant P value was obtained, the POST HOC analysis (Tukey-HSD multiple comparison tests) was performed to determine the effect of various treatments on gastric damage and MDA levels. Calculations were performed using SPSS software version 13.
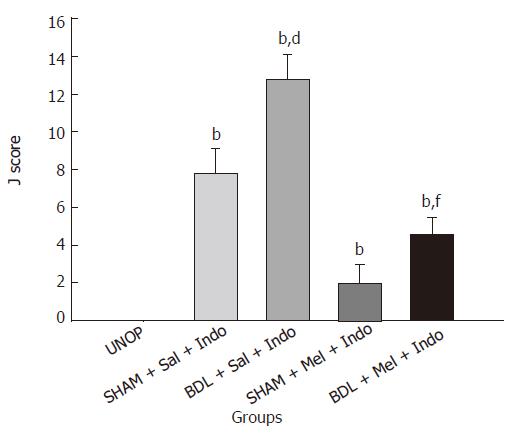
Two days after laparotomy, BDL rats showed signs of cholestasis (jaundice, dark urine and steatorrhea) confirming rise in the level of plasma bilirubin. As shown in Figure 1, 50 mg/kg of indomethacin in BDL and SHAM rats produce gastric lesions with a J-score of 12.8 ± 1.3 and 7.8 ± 1.3, respectively. These results show that gastric mucosal damage is significantly more severe in BDL compared with SHAM and UNOP (P < 0.001) animals. This means that cholestasis increased the ulcerogenic effect of indomethacin and in multistress conditions; gastric mucosal damage is significantly more severe. The effect of melatonin on indomethacin-induced gastric damage in BDL and SHAM groups has been shown in Figure 1. As shown in Figure 1, 20 mg/kg of melatonin 30 min before indomethacin reduces the ulcerogenicity of indomethacin both in BDL and SHAM rats. The J-scores in this group were 4.6 ± 0.89 and 2 ± 1, respectively. These results show that the antioxidant effect of melatonin reduces cell damage mediated by oxidative stress.
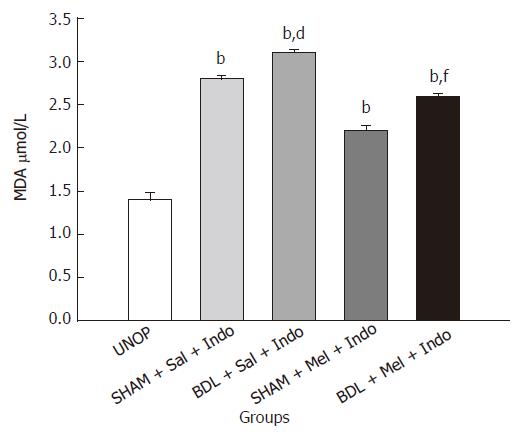
Plasma MDA levels are shown in Figure 2. MDA level is significantly higher in BDL compared with SHAM and UNOP rats (BDL = 3.1 ± 0.04; SHAM = 2.8 ± 0.04; UNOP = 1.4 ± 0.08; P < 0.001). These results show that the level of MDA is dependent on the number of stressful conditions. The effect of melatonin on MDA level in BDL and SHAM groups has been shown in Figure 2. As shown in Figure 2, 20 mg/kg of melatonin 30 min before indomethacin reduced the lipid peroxidation and MDA levels both in BDL and SHAM groups (BDL = 2.6 ± 0.03; SHAM = 2.2 ± 0.06; P < 0.001).
Oxidative stress caused by reactive oxygen species (ROS) damaged cellular lipids, proteins and DNA and is widely recognized as one of the causes of the development of chronic disease. Under normal circumstances, the levels of ROS are low enough to be effectively removed by the natural defense mechanisms of the cell. There are, however, many conditions that enhance the production of ROS to such an extent that cellular defenses are overwhelmed and the cell injured[19]. The evaluation of biomarkers of cellular stress in conditions mediated by oxidative compounds could help to prevent appearance and development of oxidative stress-related diseases. Lipid peroxidation is considered to be important in the development of atherosclerosis, to be involved in aging and other clinical disorders, such as cancer, cardiovascular and liver diseases. An important step in the degradation of cell membranes is the reaction of ROS with double bound polyunsaturated fatty acids to yield lipid hydroperoxide (as primary products). On breakdown of such hydroperoxides a great variety of aldehydes can be formed (as secondary products). MDA, a three carbon compound formed by scission of peroxidized polyunsaturated fatty acids, is one of the main secondary products of lipid peroxidation[16,22]. MDA is reactive toward amino groups of protein and nucleic acid and have cytotoxic and mutagenic effects. Since MDA has been found elevated in various diseases thought to be related to free radical damage, it has been widely used as an index of lipid peroxidation in biological and medical sciences[20,22]. Determination of lipid peroxidation products in the body fluids can be used as a diagnostic tool for detecting increased levels of ROS. The most popular method of MDA detection is derivatization of MDA with different chemicals such as TBA and DNPH (2, 4-dinitrophenylhydrazine) and conversion into derivatives to allow more specific estimation of this compound. We used the HPLC method suggested by Agarwal, a rapid and reproducible method. In this method, protein was not precipitated and samples were derivatized after addition of BHT, and extraction of derivatized sample with n-butanol, found to be a suitable extracting solvent[20]. The plasma MDA levels and J. scores in both BDL animals treated with indomethacin and SHAM animals treated with indomethacin groups were greater than UNOP animals (P < 0.001). The plasma MDA levels and J-score in BDL rats treated with indomethacin were greater than SHAM animals treated with indomethacin (P < 0.001). The plasma MDA levels and J-score in BDL animals pretreated with melatonin and treated with indomethacin and SHAM animals with melatonin and treated with indomethacin were lower than BDL animals treated with indomethacin and SHAM animals treated with indomethacin. In conclusion, our results suggest that: cholestasis can increase the indomethacin-induced gastric mucosal damage and plasma MDA level. Melatonin is an efficient agent in reducing the negative effect of lipid peroxidation and reduces cell damage by oxidative stress in multistress conditions. According to the calculated detection limit (0.101 μmol/L) and average of recovery (99%) for MDA, sensitive methods for the determination of MDA level in plasma have been used.
S- Editor Wang GP L- Editor Alpini GD E- Editor Bi L
| 1. | Galunska B, Marazova K, Tankova T, Popov A, Frangov P, Krushkov I, Di Massa A. Effects of paracetamol and propacetamol on gastric mucosal damage and gastric lipid peroxidation caused by acetylsalicylic acid (ASA) in rats. Pharmacol Res. 2002;46:141-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 2. | Samini M, Dehpour AR, Hosseinali Izad E. Cholestasis potentiates mefenamic acid-induced gastric mucosal damage in rats. Pharm Pharmacol Commun. 1999;5:463-465. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 3. | Pohle T, Brzozowski T, Becker JC, Van der Voort IR, Markmann A, Konturek SJ, Moniczewski A, Domschke W, Konturek JW. Role of reactive oxygen metabolites in aspirin-induced gastric damage in humans: gastroprotection by vitamin C. Aliment Pharmacol Ther. 2001;15:677-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 75] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | Sim AS, Salonikas C, Naidoo D, Wilcken DE. Improved method for plasma malondialdehyde measurement by high-performance liquid chromatography using methyl malondialdehyde as an internal standard. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;785:337-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 95] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 5. | Dehpour AR, Mani AR, Amanlou M, Nahavandi A, Amanpour S, Bahadori M. Naloxone is protective against indomethacin-induced gastric damage in cholestatic rats. J Gastroenterol. 1999;34:178-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Reiter RJ. Antioxidant actions of melatonin. Adv Pharmacol. 1997;38:103-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 134] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 7. | Pieri C, Marra M, Moroni F, Recchioni R, Marcheselli F. Melatonin: a peroxyl radical scavenger more effective than vitamin E. Life Sci. 1994;55:PL271-PL276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 493] [Cited by in RCA: 484] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 8. | Reiter RJ, Tan DX, Osuna C, Gitto E. Actions of melatonin in the reduction of oxidative stress. A review. J Biomed Sci. 2000;7:444-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 763] [Cited by in RCA: 779] [Article Influence: 31.2] [Reference Citation Analysis (1)] |
| 9. | Pieri C, Moroni F, Marra M, Marcheselli F, Recchioni R. Melatonin is an efficient antioxidant. Arch Gerontol Geriatr. 1995;20:159-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 78] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 10. | López PM, Fiñana IT, De Agueda MC, Sánchez EC, Muñoz MC, Alvarez JP, De La Torre Lozano EJ. Protective effect of melatonin against oxidative stress induced by ligature of extra-hepatic biliary duct in rats: comparison with the effect of S-adenosyl-L-methionine. J Pineal Res. 2000;28:143-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Montilla P, Cruz A, Padillo FJ, Túnez I, Gascon F, Muñoz MC, Gómez M, Pera C. Melatonin versus vitamin E as protective treatment against oxidative stress after extra-hepatic bile duct ligation in rats. J Pineal Res. 2001;31:138-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 114] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 12. | Padillo FJ, Cruz A, Navarrete C, Bujalance I, Briceño J, Gallardo JI, Marchal T, Caballero R, Túnez I, Muntané J. Melatonin prevents oxidative stress and hepatocyte cell death induced by experimental cholestasis. Free Radic Res. 2004;38:697-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 54] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Esrefoglu M, Gül M, Emre MH, Polat A, Selimoglu MA. Protective effect of low dose of melatonin against cholestatic oxidative stress after common bile duct ligation in rats. World J Gastroenterol. 2005;11:1951-1956. [PubMed] |
| 14. | Ohta Y, Kongo M, Kishikawa T. Melatonin exerts a therapeutic effect on cholestatic liver injury in rats with bile duct ligation. J Pineal Res. 2003;34:119-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Moezi L, Rezayat M, Samini M, Shafaroodi H, Mehr SE, Ebrahimkhani MR, Dehpour AR. Potentiation of anandamide effects in mesenteric beds isolated from bile duct-ligated rats: role of nitric oxide. Eur J Pharmacol. 2004;486:53-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Khoschsorur GA, Winklhofer-Roob BM, Rabl H, Auer TH, Peng Z, Schaur RJ. Evaluation of a sensitive HPLC method for the determination of malondialdehyde, and application of the method to different biological materials. Chromatographia. 2000;52:181-184. [RCA] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 109] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 17. | Ohta Y, Kongo M, Kishikawa T. Therapeutic effect of melatonin on cholestatic liver injury in rats with bile duct ligation. Adv Exp Med Biol. 2003;527:559-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Morley JE, Levine AS, Silvis SE. Endogenous opiates and stress ulceration. Life Sci. 1982;31:693-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Samini M, Moezi L, Jabarizadeh N, Tavakolifar B, Shafaroodi H, Dehpour AR. Evidences for involvement of nitric oxide in the gastroprotective effect of bromocriptine and cyclosporin A on water immersion stress-induced gastric lesions. Pharmacol Res. 2002;46:519-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Agarwal R, Chase SD. Rapid, fluorimetric-liquid chromatographic determination of malondialdehyde in biological samples. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;775:121-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 225] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 21. | Miller JC, Miller JN. Statistics and Chemometrics for analytical chemistry. 2nd ed. Chilchester: Halsted Press 1988; 115-117. |
| 22. | Mateos R, Goya L, Bravo L. Determination of malondialdehyde by liquid chromatography as the 2,4-dinitrophenylhydrazone derivative: a marker for oxidative stress in cell cultures of human hepatoma HepG2. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;805:33-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 3.4] [Reference Citation Analysis (0)] |