Published online Dec 7, 2006. doi: 10.3748/wjg.v12.i45.7388
Revised: September 28, 2006
Accepted: October 25, 2006
Published online: December 7, 2006
AIM: To investigate the protein and mRNA expression of semaphorin 6D in gastric carcinoma and its significance.
METHODS: The protein and mRNA expression of semaphorin 6D was detected by semi-quantitative rever-se transcription PCR and Western blotting respectively in 30 cases of gastric carcinoma and normal gastric mucosa.
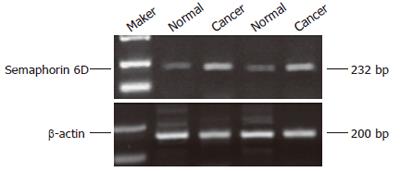
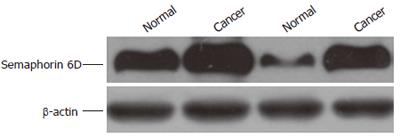
RESULTS: The protein and mRNA expression of semaphorin 6D in gastric carcinoma was significantly higher than that in normal gastric mucosa (0.24 ± 0.06 vs 0.19 ± 0.07, 0.26 ± 0.09 vs 0.20 ± 0.10, P < 0.05).
CONCLUSION: Semaphorin 6D may play an important role in the occurrence and development of gastric carcinoma, and is related to tumor angiogenesis.
- Citation: Zhao XY, Chen L, Xu Q, Li YH. Expression of semaphorin 6D in gastric carcinoma and its significance. World J Gastroenterol 2006; 12(45): 7388-7390
- URL: https://www.wjgnet.com/1007-9327/full/v12/i45/7388.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i45.7388
The semaphorin family is characterized by a phylogene-tically conserved sema domain in the extracellular region[1]. This family has been subdivided into eight groups, which play a crucial role in formation of nervous system, embryogenesis, angiogenesis, immunoreaction, and carcinogenesis[1-5]. Semaphorin 6D is a novel member of class 6 semaphorin gene, mapped on the chromosome 2, and it is grouped into five isoforms through different splicing[6]. At present, little is known about the function of semaphorin 6D in carcinogenesis. Therefore, we detected the protein and mRNA expression of semaphorin 6D in gastric carcinoma and normal gastric mucosa by semi-quantitative RT-PCR and Western blotting in order to explore the expression of semaphorin 6D in gastric carcinoma and its significance.
Thirty fresh gastric carcinoma specimens and thirty normal gastric mucosa specimens were obtained from patients who underwent surgery for gastric carcinoma between April 2006 and August 2006 in General Hospital of Chinese PLA and analyzed. The diagnosis of gastric carcinoma was confirmed by pathological examination. The patients with gastric carcinoma consisted of 11 women and 19 men. Their mean age was 54 years and ranged from 32 to 74 years. Of the 30 patients, 13 showed moderate differentiation, 17 poor differentiation.
Total mRNA was isolated by Trizol reagent according to the procedure of supplier (BioDev-tech, Beijing, China). The concentration was determined by measuring the absorbance at 260 nm and using the following equation: one optical density unit = 40 mg of RNA/mL. A 1.5 μg aliquot of total RNA from each specimen was reverse -transcribed into single-strand cDNA using oligo (dT)16 primer for 2 h at 37°C. Each single-strand cDNA was used for subsequent PCR amplification of semaphorin 6D and β-actin with the latter used as a quantitative control. PCR was carried out in a reaction volume of 50 μL: an initial denaturation for 5 min at 95°C, followed by 37 cycles at 94°C for 50 s, at 55°C for 50 s, and at 72°C for 1 min, then a final extension at 72°C for 10 min on the authorized thermal cycler for PCR. The primer sequences used for amplification were 5’-CTCAGTCGCTGTGAGCGTTAT-3’ and 5’-CAGATGTTGGACCGCCAAATA-3’ for samephorin 6D, 5’-CGCACCACTGGCATTGTCAT -3’ and 5’-TTCTCCTTGATGTCACGCAC -3’ for β-actin. The primer sequences were synthesized by Beijing Genomics Institute (China). The PCR products were resolved in 2% agarose gels and visualized by staining with ethidium bromide. To quantify the PCR products, the bands representing amplified products were analyzed by Quantity One Analysis Software (BIO-RAD Co., USA).
Expression of the semaphorin 6D protein was detected using the Western blot method. After washed in ice-cold PBS, the samples were finely minced and suspended in ice-cold homogenization buffer (2 mL/g of tissue) containing protease inhibitors to minimize protein degradation. The suspension was homogenized, then centrifuged at 12 000 ×g for 30 min at 4°C to remove nuclei and cell debris. The supernatant (total protein extract) was collected. An equal amount (50 μg) of proteins was run on a 10% SDS-PAGE gel and electrotransferred onto hybond-polyvinylidene difluoride membranes (Amersham, Arlington Heights, USA). The membranes were blocked for 2 h at room temperature, followed by incubation with 3 μg/mL primary anti-semaphorin antibody (R&D system, Minneapolis, USA) at 4°C overnight. The primary antibody was diluted in TBST containing fat-free milk. After three washes (10 min each time) in TBST, membrane was incubated in peroxidate-conjugated second antibody (Sigma, St. Louis, USA) diluted 1:800 at room temperature for 1 h. Immunoreactive proteins were visualized on autoradiogram using ECL Western blotting detection reagents (Amershan Pharmacia, Uppsala, Sweden) and exposed to X-Omat BT film (Kodak, New York, USA). Bands were analyzed by Quantity One Analysis Software normalized with respect to β-actin as an internal control.
Results were expressed as mean ± SD. The statistical differences between different groups were analyzed by paired t-test. P < 0.05 was considered statistically significant. All analyses were performed by SPSS12.0 statistical software.
Two percent of agarose gel electrophoresis showed a 232bp semaphorin 6D fragment by RT-PCR amplification from gastric caner and normal gastric mucosa specimens (Figure 1). The semaphorin 6D mRNA amplification was successful in all tissues. The expression level was 0.24 ± 0.06 in tumor, much higher than that in normal gastric mucosa (0.19 ± 0.07, P < 0.05).
The affinity-purified anti-semaphorin 6D antibody detected a major band at 100 kDa in protein extracts from all samples tested (Figure 2). The expression was 0.26 ± 0.09 in tumor, much higher than that in normal gastric mucosa (0.20 ± 0.10, P < 0.05), which was consistent with that of RT-PCR.
The semaphorin family is characterized by a phyloge-netically conserved sema domain in the extracellular region. On the basis of additional structural features, such as the presence or absence of transmembrane domains, Ig-like domains, thrombospondin repeats, and glycophosphatidylinositol linkage sites, the family has been subdivided into eight groups, including virally derived proteins[1]. Despite the fact that a number of semaphorins have been known to play a crucial role in the hard wiring of the nervous system, including fasciculation, axon branching, and target selection as axonal guidance cues[2,3]. There is increasing evidence that semaphorins play a significant role in angiogenesis. For example, secreted class III semaphorins, regulating axonal growth during the development of central nervous system[7,8], can initiate signaling events in a variety of tissues that influence vascular morphogenesis and endothelial cell motility[9-11]. It was reported that semaphorin 4D is highly expressed in endothelial cells and promotes endothelial cell migration and tubulogenesis[12].
Gastric carcinoma is one of the most common malignant tumors. As other malignant tumors, its growth and metastasis require induction of angiogenesis (the growth and remodeling of new blood vessels from a preexisting vascular network) to ensure the delivery of oxygen, nutrients, and growth factors to rapidly divide transformed cells and to provide tumor cell access to systemic circulation[13-15]. Without the ability to induce angiogenesis, most neoplasms would fail to grow (> 2 mm in diameter) or metastasize[15]. Semaphorin 6D is a novel class 6 semaphorin gene, mapped on the chromosome 2, and can be grouped into five isoforms through different splicing, including one short isoform (only consisting of extrocellular domain) and four long isoforms[6]. It was reported that semaphorin 6D can inhibit axonal extension of NGF-differentiated PC12 cells and to collapse growth cones of chick DRG and rat hippocampal neurons[6]. It was also reported that semaphorin 6D also can attract or inhibit epithelial cell migration depending on different special regions through different receptor complex in cardiac morphogenesis of chick embryos[16], suggesting that semaphorin 6D may regulate angiogenesis in vivo, and increase the intriguing possibility, thus playing a role in tumor-induced angiogenesis, including gastric carcinoma. Therefore, we detected the protein and mRNA expression of semaphorin 6D in gastric carcinoma and normal gastric mucosa by semi-quantity RT-PCR and Western blotting. The results showed that the expression of semaphorin 6D was significantly higher in gastric carcinoma than in normal gastric mucosa, suggesting that semaphorin 6D plays an important role in the development of gastric carcinoma. Semaphorin 6D may induce tumor angiogenesis, thereby enhancing tumor growth, survival, and metastasis.
In conclusion, this is the first time to investigate the expression of semaphorin 6D in gastric carcinoma and its clinical significance. The results of our study shed some light on the pathogenesis of gastric carcinoma, and may represent a new therapeutic target for gastric carcinoma treatment.
S- Editor Wang J L- Editor Wang XL E- Editor Ma WH
| 1. | Gherardi E, Love CA, Esnouf RM, Jones EY. The sema domain. Curr Opin Struct Biol. 2004;14:669-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 113] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 2. | Liu Y, Halloran MC. Central and peripheral axon branches from one neuron are guided differentially by Semaphorin3D and transient axonal glycoprotein-1. J Neurosci. 2005;25:10556-10563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 68] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 3. | Bouzioukh F, Daoudal G, Falk J, Debanne D, Rougon G, Castellani V. Semaphorin3A regulates synaptic function of differentiated hippocampal neurons. Eur J Neurosci. 2006;23:2247-2254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 62] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 4. | Czopik AK, Bynoe MS, Palm N, Raine CS, Medzhitov R. Semaphorin 7A is a negative regulator of T cell responses. Immunity. 2006;24:591-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 94] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 5. | Basile JR, Castilho RM, Williams VP, Gutkind JS. Semaphorin 4D provides a link between axon guidance processes and tumor-induced angiogenesis. Proc Natl Acad Sci USA. 2006;103:9017-9022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 169] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 6. | Qu X, Wei H, Zhai Y, Que H, Chen Q, Tang F, Wu Y, Xing G, Zhu Y, Liu S. Identification, characterization, and functional study of the two novel human members of the semaphorin gene family. J Biol Chem. 2002;277:35574-35585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274:1123-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2411] [Cited by in RCA: 2472] [Article Influence: 85.2] [Reference Citation Analysis (0)] |
| 8. | Raper JA. Semaphorins and their receptors in vertebrates and invertebrates. Curr Opin Neurobiol. 2000;10:88-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 345] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 9. | Carmeliet P, Tessier-Lavigne M. Common mechanisms of nerve and blood vessel wiring. Nature. 2005;436:193-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 742] [Cited by in RCA: 793] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 10. | Autiero M, De Smet F, Claes F, Carmeliet P. Role of neural guidance signals in blood vessel navigation. Cardiovasc Res. 2005;65:629-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 68] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Miao HQ, Soker S, Feiner L, Alonso JL, Raper JA, Klagsbrun M. Neuropilin-1 mediates collapsin-1/semaphorin III inhibition of endothelial cell motility: functional competition of collapsin-1 and vascular endothelial growth factor-165. J Cell Biol. 1999;146:233-242. [PubMed] |
| 12. | Basile JR, Barac A, Zhu T, Guan KL, Gutkind JS. Class IV semaphorins promote angiogenesis by stimulating Rho-initiated pathways through plexin-B. Cancer Res. 2004;64:5212-5224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 195] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 13. | Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2432] [Cited by in RCA: 2467] [Article Influence: 112.1] [Reference Citation Analysis (0)] |
| 14. | Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2935] [Cited by in RCA: 2882] [Article Influence: 115.3] [Reference Citation Analysis (0)] |
| 15. | Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5621] [Cited by in RCA: 5531] [Article Influence: 184.4] [Reference Citation Analysis (0)] |
| 16. | Toyofuku T, Zhang H, Kumanogoh A, Takegahara N, Suto F, Kamei J, Aoki K, Yabuki M, Hori M, Fujisawa H. Dual roles of Sema6D in cardiac morphogenesis through region-specific association of its receptor, Plexin-A1, with off-track and vascular endothelial growth factor receptor type 2. Genes Dev. 2004;18:435-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 235] [Article Influence: 11.2] [Reference Citation Analysis (0)] |