INTRODUCTION
Persistent hyperinsulinemic hypoglycemia (PHH) caused by functionally defectice β-cells in the setting of a nesidioblastosis is the most common pathological substrate in newborns, whereas in adults, PHH is usually caused by solitary insulinomas[1-3]. Several genetic abnormalities were identified as the causes of PHH in infancy. The most important mutations are in the β-cell sulfonylurea receptor (SUR1) gene and encoding proteins composing the ATP-sensitive potassium channel in the cell membrane of the β-cell (Kir6.2)[4,5]. Loss of function due to mutations of the glucokinase (GCK) and glutamat dehydrogenase (GLUD1) genes were also identified as possible causes of PHH of infancy[6-8]. The morphologic substrate of these molecular changes are either diffuse β-cell hypertrophy (diffuse nesidioblastosis) or focal β-cell hypertrophy and β-cell hyperplasia (focal nesidioblastosis)[4,9]. The term nesidioblastosis for describing the lesions of the endocrine pancreas in neonates was first used by Yakovac[10] in 1971, when he described 12 infants with PHH.
Establishing the diagnosis of nesidioblastosis in an adult is a great challenge in endocrinology. To date, it is not possible to diagnose diffuse nesidioblastosis in adults on clinical grounds, since imaging techniques or detection of a specific causative mutation are of no help. The fasten test for the diagnosis of nesidioblastosis has been controversially discussed. Up to now, the diagnosis is based on (1) exclusion of an insulinoma by all means of clinical diagnostic procedures and (2) pathological analysis of the pancreatic tissue specimens. A combination of various histopathological criteria, marked as major and minor criteria, has recently been published to establish the diagnosis of a diffuse adult nesidioblastosis[11].
Since the first reported case of an adult nesidioblastosis in 1975, less than 100 patients have been described in the literature. Most of them were reported in individual case studies. Reports of PHH with known diabetes mellitus are extremely rare[12-15]. In our hospital, diffuse nesidioblastosis has been diagnosed in 4 out of 137 patients suffering from PHH. A coincidence of PHH with diabetes was diagnosed in only one patient. The aim of our report is to describe this patient in detail concerning (1) clinical presentation, (2) mutational analysis of the MEN1 and Mody 2/3 genes, (3) pathology of the pancreatic specimens. The diagnostic procedures and the surgical treatment strategies are discussed on the basis of the presented case.
CASE REPORT
Clinical presentation
A 40-year-old man (170 cm in height; BMI: 24.9) diagnosed with organic hyperinsulinism was admitted to our hospital. The previous medical history was marked by a primarily insulin-independent diabetes (type II). Insulin treatment has been required over the past 6 years. At the age of 35, he developed an idiopathic facial nerve pareses and brainstem encepahlitis of unknown etiology. Family history showed no pathologic findings, except diabetes of a grandmother. On the onset of diabetes, his blood sugar rose to 3800 mg/L with elevated HbA1c (8.0%). The patient was started on insulin treatment. During three hospital admissions for hyperglycemia over a 5-year period, blood sugar levels were often higher than 4000 mg/L and he was treated with insulin. Starting in 2005, the insulin treatment began to reduce and two months later discontinued, because of normalized serum glucose levels. He was admitted to hospital for symptoms of hyperadrenergic reactions and neuroglycopenic symptoms of confusion and somnolence due to hypoglycemia with blood sugar levels of 250 mg/L.
Diagnosis with a pathological fasting test indicated hypoglycemia. Blood sugar levels were repeatedly reduced to 300 mg/L with typical neuroglycopenic symptoms. The insulin level was up to 93.3 mU/L with a raised insulin index of 2-3. Exogenous insulin intake could be excluded by C-peptide levels. Unfortunately, the real C-peptide level could not be specified because of insufficient terms of transport (external laboratory). Insulin antibodies were negative. Attempts to localize an insulinoma with computed tomography (CT), ultrasound (US) of the abdomen and endoscopic ultrasound (EUS) were unsuccessful.
Laparotomy was performed and the pancreas was completely exposed and investigated by bimanual palpation and intraoperative ultrasound. A spleen preserving pancreatic-tail resection was performed because of a suspected small tumor in the tail of the pancreas. The patient’s postoperative course was uneventful. Postoperatively, he displayed no signs of recurrent hypoglycemia. The blood sugar levels measured rose up to 1660-2430 mg/L, therefore low dose insulin application was necessary. The patient was discharged on the seventh postoperative day.
The patient and his family doctor reported recurrent diabetes metabolism over the previous 5 mo during a nine month follow-up after surgery (blood sugar levels: 300-2420 mg/L depending on insulin intake). Unfortunately, a reassessment of the diabetes was declined by the patient at the time.
Mutational analysis
Genetic sequence analysis of the exons 2-10 of the MEN1 gene on chromosome 11q13 as previously described[16,17], was performed for differential diagnosis. The genetic make-up ruled out a molecular germline mutation in the MEN1 gene. Additional genetic sequence analyses of the glukokinase-gene of exon 1A-10 on chromosome 7p15-p13 were performed. Mutations pointing to maturity diabetes of the young Typ 2 were excluded. In addition, genetic sequence analyses of exons 1-10 of the hepatic nuclear factor 1 alpha gene for MODY Typ 3 were negative.
Histopathological analysis
Pathological examinations were done using serial sections from different regions of the specimens which were immunohistochemically stained, by the avidin-biotin-complex (ABC) method with a monoclonal antibody against insulin (BioGenex, San Ramon, Canada, primary dilution 1:40) and polyclonal antibodies against glucagon (BioGenex, dilution: 1:60), somatostatin (Dako, Glostrup, Denmark, dilution: 1:200) and pancreatic polypeptide (DAKO, dilution: 1:5000). Appropriate controls were performed in order to ascertain the staining specificity. Histological and immunostained pancreatic sections were analyzed by three different pathologists in a double-blind fashion. Histological criteria for nesidioblastosis in adults were used to evaluate the sections[11]. Age- and sex-matched control pancreatic specimens were used as references.
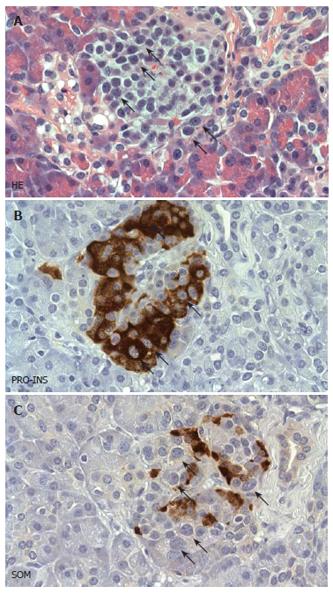
Gross examination and step sectioning of the pancreatic samples excluded the presence of an endocrine tumor. The lobular architecture of the exocrine parenchyma was preserved. The pancreatic duct was patent and of normal size. There was no evidence of amyloid deposits or a quantitative disturbance of beta-cells. A consistent finding was the presence of multiple enlarged islet cells, whose arrangement often produced a lobulated islet pattern. Cytologically, a considerable subpopulation of endocrine cells showed enlarged and hyperchromatic nuclei (Figure 1A). These cytological changes were seen in more than 2/3 of the islets. Using immunohistochemistry and subsequent staining with hematoxylin, the cytologically conspicuous endocrine cells were identified as beta-cells (Figure 1B). In contrast, glucagon, somatostatin and pancreatic polypeptide cells did not reveal any cytologic abnormalities (Figure 1C). The subcellular distribution of insulin was regular and apparently equal amounts of C-Peptide were seen. There was no evidence of an expression of the nuclear proliferation antigen ki-67 within the islets. According to this histopathological analysis the patient fulfilled the recently described “major criteria” of a diffuse nesidioblastosis in adults[11].
Figure 1 Histopathological features of diffuse nesidioblastosis.
A: HE stained sections demonstrating a prominent lobulation of an islet. Some of the endocrine cells showing hyperchromatic and enlarged nuclei are labelled with arrows. B, C: Adjacent section analysis demonstrating cytoplasmic positivity for proinsulin (PRO-INS) in those endocrine cells with hyperchromatic nuclei (arrows in B). In contrast, these cells are negative for somatostatin (SOM) (arrows in C).
DISCUSSION
Nesidioblastosis was detected and diagnosed in 4 patients out of a series of 137 patients treated for organic hyperinsulinism at our hospital. One of them was diagnosed by clinically reversing a highly insulin-dependant diabetes. Diagnosis of nesidioblastosis was established by (1) exclusion of an insulinoma by all means of clinical diagnostic procedures and (2) by pathological examination revealing the characteristic signs of a diffuse nesidioblastosis.
Preoperative localization by CT-scan, abdominal ultrasound and endoscopic ultrasound (EUS) failed to detect an endocrine tumor. An indication for surgery was based on hypoglycemia in the presence of elevated insulin levels and excluding exogene insulin intake by C-peptide levels and screening for sulfonylureas.
After completely exposing the pancreas and investigation by bimanual palpation and intraoperative ultrasound, a small tumor in the tail of the pancreas was suspected and a spleen preserving pancreatic-tail resection was performed. Nesidioblastosis was diagnosed postoperatively by the pathologist according to the recently published major and minor criteria for nesidioblastosis in adults[11].
Our knowledge of the pathogenesis of PHH in infants has expanded in recent years. Some genetic abnormalities underlie the disease. The most important genetic defects are inactivating mutations on chromosome 11 (SUR1 and Kir6.2)[4,5]. Gain-of-function mutations of the glucokinase gene (GCK) and glutamate dehydrogenase genes are infrequent (GLUD1)[11]. Because of the patient’s young age, a subgroup of diabetes mellitus, a maturity onset diabetes of the youth (MODY), should be discussed for differential diagnosis. So far 6 different types of MODY have been diagnosed. Glucokinase plays a critical role in the insulin secretion in β-cells. Heterozygous mutations in the glucokinase-encoding gene (GCK) results in the reduction of encymatic activity (MODY 2)[18,19]. Loss of function mutations in GCK could be ruled out as a possible reason for hyperglycemia in our patient.
The diagnosis and treatment of an adult nesidio-blastosis still remain an open issue. There are no imaging techniques to differentiate focal from nonfocal (diffuse) organic hyperinsulinism. Frequently, insulin-producing tumors are less than 10 mm in diameter and may therefore escape preoperative detection. Additionally, preoperative imaging as EUS can yield misleading findings in the pancreas[20]. An intra-arterial calcium stimulation test may be able to differentiate focal hyperactive β-cells from diffuse hypertophic β-cells[21-23]. Due to the failure of the modern imaging techniques in these patients, the localization of pathologic increased insulin secretion would be a diagnostic tool for the surgeon to help avoid blind pancreatic resection. The treatment of adult nesidioblastosis is surgical resection. However, the line between ‘too much’ and ‘too less’ resection, with the risk of endocrine and exocrine pancreatic insufficiency and recurrent hypoglycemia is very narrow. Nesiodioblastosis has to be considered in all cases without localized insulinoma because it has been recently shown that this disease can be present in up to 4% of patients with PHH[11]. In the case of recurrence, a secondary surgical intervention with further pancreatic resection is necessary. In our case, hypoglycemia may be life threatening, but the risk of lifetime diabetes should be considered as well. Nowadays, recurrent pancreatic resections can be performed with low risk in specialized centers.
The present case illustrates a very rare coincidence of diabetes and adult nesidioblastosis. Up to now, only four cases with organic hyperinsulinism and diabetes have been reported[12-15]. Our case is the second description of a concomitant adult nesidioblastosis and diabetes type II. In summary, diabetes can be reversed by functionally defective hypertrophic β-cells. In the case of reversing diabetes, an organic hyperinsulinemic hypoglycemia should be considered as a differential diagnosis.