Published online Nov 21, 2006. doi: 10.3748/wjg.v12.i43.6926
Revised: September 12, 2006
Accepted: September 19, 2006
Published online: November 21, 2006
Evidence has accumulated to suggest an important role of ethanol and/or its metabolites in the pathogenesis of alcohol-related liver disease. In this review, the fibrogenic effects of ethanol and its metabolites on hepatic stellate cells (HSCs) are discussed. In brief, ethanol interferes with retinoid metabolism and its signaling, induces the release of fibrogenic cytokines such as transforming growth factor β-1 (TGFβ-1) from HSCs, up-regulates the gene expression of collagen I and enhances type I collagen protein production by HSCs. Ethanol further perpetuates an activated HSC phenotype through extracellular matrix remodeling. The underlying pathophysiologic mechanisms by which ethanol exerts these pro-fibrogenic effects on HSCs are reviewed.
- Citation: Wang JH, Batey RG, George J. Role of ethanol in the regulation of hepatic stellate cell Function. World J Gastroenterol 2006; 12(43): 6926-6932
- URL: https://www.wjgnet.com/1007-9327/full/v12/i43/6926.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i43.6926
Ethanol abuse is a leading cause for morbidity and mortality throughout the world. It affects many organ systems, most notably the liver causing both acute and chronic liver disease, and the central nervous system[1-3]. Hepatic cirrhosis resulting from alcohol abuse is one of the principal causes of liver-related morbidity and mortality. In the liver, excess ethanol leads to three pathologically distinct disorders, namely fatty liver (alcohol-associated hepatic steatosis), alcoholic hepatitis and cirrhosis. Alcohol-associated hepatic steatosis is the most common form of liver injury and is reversible with abstinence[3-5]. More serious forms of alcoholic liver disease (ALD) include alcoholic hepatitis characterized by persistent inflammation of the liver, and cirrhosis, characterized by progressive hepatic fibrosis. The pathogenesis of ALD is poorly understood, in part because no simple animal model exists that reproduces the full spectrum of the human disease, including the development of cirrhosis[1,4]. In addition, there is considerable variation among individuals in their susceptibility to ALD, so that among people drinking similar amounts, only a proportion develops cirrhosis[1,3-5].
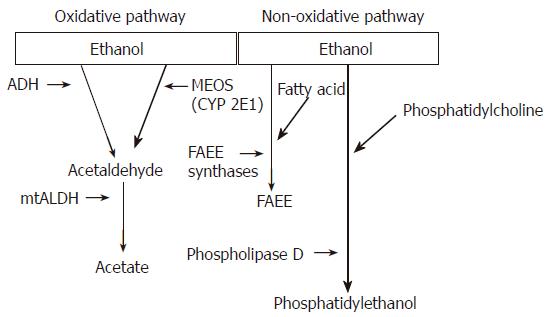
Almost all ingested ethanol is metabolized in the liver. Two major enzyme systems, namely the oxidative and non-oxidative pathways, mediate the initial phase of ethanol metabolism[1,5] (Figure 1). The oxidative pathway comprises the alcohol dehydrogenases (ADH) and members of the cytochrome P450 system (predominantly CYP2E1)[5-7]. This pathway generates acetaldehyde. Acetaldehyde is subsequently metabolized to acetate via the mitochondrial enzyme acetaldehyde dehydrogenase (ALDH). Although acetaldehyde is oxidized to acetate by ALDH, the kinetics of this reaction is sufficiently slow to allow for the accumulation of acetaldehyde in humans or animals consuming alcohol[1,2,5]. The non-oxidative pathway of ethanol metabolism involves the esterification of ethanol with fatty acids to form fatty acid ethyl esters (FAEE), a reaction catalyzed by FAEE synthases[1,5].
Ethanol and its metabolites including acetaldehyde cause liver damage through several interrelated pathways[1,2,8,9]. The oxidation of ethanol is associated with a change in hepatocyte redox homeostasis which can lead to a number of metabolic disorders including lactic acidosis, hyperlipidaemia and hyperuricaemia. Chronic ethanol consumption does not influence ADH activity, but has a profound stimulatory effect on microsomal enzymes, particularly CYP2E1[1,2]. This is in part responsible for the development in alcoholic liver diseases, a rise in oxygen consumption, the excessive production of free radicals and an increase in the metabolism of ethanol, vitamin A and testosterone. Ethanol and acetaldehyde have deleterious effects both direct and indirect, for example by generating reactive oxygen species (ROS) and causing damage to the intestinal mucosal barrier[1,10]. Cellular oxidative stress that is caused by the relative imbalance between free radical generation and insufficient anti-oxidant defense mechanisms, including reductions in glutathione, vitamin E and phosphatidylcholine, may be a principal mediator for the progression of alcoholic liver disease[1,2,10].
Steatosis, hepatitis and fibrosis seen in persons with ALD are a consequence of complex pathophysiological events involving various cell types within the liver including neutrophils, sinusoidal endothelial cells (SECs), Kupffer cells (KCs), hepatic stellate cells (HSCs) and hepatocytes. Recently, many studies have demonstrated that ethanol and its metabolites including acetaldehyde directly activate HSCs, the principal fibroblastic cell type within the liver[8,9,11]. Ethanol and acetaldehyde directly promote the production of transforming growth factor beta-1 (TGFβ-1) and several extracellular matrix (ECM) constituents including type I collagen by HSCs[8,9,11].
This article reviews recent advances in our knowledge on the effects of ethanol and its metabolites on HSCs.
A central event in liver fibrosis is the activation of HSCs, which represents a transition from a quiescent vitamin A-rich cell type to a vitamin A-deficient, proliferative, fibrogenic and contractile myofibroblast. Activated HSCs demonstrate altered cell behaviors including proliferation, chemotaxis, fibrogenesis, contractility, matrix degradation, retinoid loss, leukocyte chemotaxis and cytokine release. In total, these changes result in excess ECM deposition which is reabsorbed, culminating in the development of liver fibrosis.
HSCs derived from the intragastric ethanol infusion model of ALD demonstrate an activated phenotype including an increase in collagen I and DNA synthesis[12], expression of α-smooth muscle actin (α-SMA) and depletion of retinyl palmitate[13].
HSCs are the major site of vitamin A storage in healthy adults. Vitamin A in HSCs is in the form of retinyl esters located in cytoplasmic lipid droplets[14]. The three active forms of vitamin A, namely retinol, retinal and retinoic acid (RA) are important regulators of cell proliferation and differentiation, binding to 2 distinct families of ligand-activated transcription factors: the retinoic acid receptor (RARs: RARα, RARβ and RARγ) and the retinoid X receptor (RXR)[15]. The natural ligand for the RARs is all trans-retinoic acid (ATRA). Published data indicate that HSCs from healthy rats express mRNAs in the RARs and RXRs[16].
Nutritionally reduced levels of serum and hepatic vitamin A have been reported in persons with ALD and in animal models of the disease[17,18]. In HSCs, ethanol significantly inhibits RA production[19] and reduces the retinol level[20]. Acetaldehyde exposure results in a reduction in RARβ message and protein in HSCs[21]. There are several other possible mechanisms by which ethanol can interfere with retinoid metabolism in the liver[19], including decreased vitamin A uptake, enhanced degradation of vitamin A in the liver, enhanced vitamin A mobilization from the liver to other organs, and degradation by ethanol of RA into polar inactive metabolites via induction of cytochrome P4502E1.
The activation and differentiation of HSCs are characterized by proliferation and an increase in the production of ECM proteins together with a loss of cellular retinoids. Therefore, it is plausible that ethanol-induced RA metabolism in HSCs could play a role in the development of alcohol-related liver fibrosis and cirrhosis.
Linolenic acid ethyl esters (LAEE), one of the FAEE products of non-oxidative ethanol metabolism, may promote HSC proliferation[22]. This effect is thought to be modulated through increased cyclin E and cyclin-dependant kinase 2 (CDK2) activities[22]. Ethanol, acetaldehyde and lactate, in contrast, have no direct effect on HSC proliferation[23,24].
Ethanol induces early protein expression of α-SMA in cultured HSCs compared to controls[25,26]. Chen and colleagues[27] likewise reported that α-SMA mRNA expression in HSCs is significantly enhanced by exposure to acetaldehyde. However, Poniachik et al[24] were unable to replicate this finding. Hence, the effects of ethanol on HSC proliferation and α-SMA expression remain controversial.
HSC activation is characterized by an increase in the production of ECM, mainly collagen types I and III. In addition, HSC activation is associated with alterations in both types of collagen, matrix-degrading metalloproteinases (MMPs) and their inhibitors, tissue inhibitors of metalloproteinases (TIMPs)[28]. Failure of matrix degradation leads to ECM accumulation and progressive hepatic fibrosis[28].
Numerous studies have shown that ethanol and/or its metabolites regulate the expression of multiple components of the ECM in HSCs. Both ethanol and acetaldehyde induce α1 (I) collagen mRNA expression in HSCs[29-33] but not hepatocytes[30]. This effect is protein synthesis-independent[29]. Acetaldehyde increases the steady-stage levels of α2 (I) gene expression[21,34] and the production of type I collagen protein[29-31] in HSCs. Acetaldehyde likewise up-regulates the mRNA expression of MMP-2 and fibronectin in human HSCs[35,36].
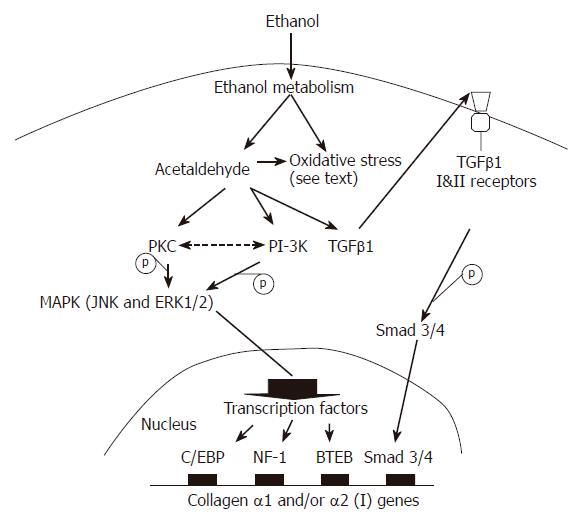
The mechanisms by which ethanol and its metabolites regulate ECM gene and/or protein expression in HSCs have not been completely elucidated (Figure 2). Several centers have reported that the MAPK and PI-3K pathways are involved[35,37,38]. Anania and colleagues[37] noted that in rat HSCs, phospho-JNK is elevated following exposure to acetaldehyde. Inhibition of JNK by curcumin at low doses reduces acetaldehyde-induced steady-state levels of endogenous α1 (I) collagen mRNA expression[37]. Phosphorylated ERK and p38 are detectable but not significantly elevated. It seems likely therefore that JNK is the principal mediator of acetaldehyde-induced α1 (I) collagen gene up-regulation in rat HSCs. These finding are consistent with those previously reported by Chen et al[38]. In contrast, in human HSCs, ERK1/2 and the PI-3K pathway appear to be triggered by acetaldehyde, leading to α2 (I) collagen and fibronectin gene up-regulation[35].
The protein kinase C (PKC) pathway may also play a role in the up-regulation of collagen gene transcription following exposure to ethanol, since PKC is upstream of ERK1/2 and JNK[35,39,40]. Acetaldehyde-elicited α2 (I) collagen and fibronectin gene expression in human HSCs is inhibited by calphostin C (a PKC inhibitor). This PKC inhibitor also reduces the enhancing effect of acetaldehyde on α1 (I) collagen mRNA expression in cultured mouse and human HSCs[35,39]. Other experiments noted that acetaldehyde increases the translocation of PKC activity to membrane fractions[39] and both α1 (I) and α2 (I) collagen gene transcription in a calcium-independent manner[39].
The modulation of gene expression in response to an exogenous or endogenous stimulus occurs through alterations in any one of the steps of gene transcription, mRNA stability, protein translation or protein degradation. Transcription factors are generally classified according to the conserved motifs within either their activation- or DNA- binding domains[41,42]. The binding of transcription factors at DNA-binding sites brings them into proximity with RNA polymerase II and components of the transcription complex that assemble in the 5'untranscribed region of genes[42,42]. Transcription factors are then able to exert either a positive or a negative influence on the rate at which the transcription complex transcribes the gene of interest. Transcriptional control of acetaldehyde-induced type I collagen gene expression might be regulated through CCAAT/enhancer-binding proteins (C/EBP), nuclear factor-I (NF-I), basic transcription element binding (BTEB) protein as well as activating protein-1 (AP-1)[34,38,43,44]. The precise mechanisms however, remain to be clarified.
A C/EBP binding site is present in the α1 (I) collagen promoter between -365 and -335 of the transcription start site[44]. Transfection of the α1 (I) collagen promoter mutated at the C/EBP binding site results in unresponsiveness to acetaldehyde, indicating that this site is essential for the collagen gene transcription effect of acetaldehyde[34,44]. C/EBP consists of 6 members. The principal form present in activated HSCs is C/EBPβ[44]. In turn, four C/EBPβ isoforms with approximate molecular weights of 45, 43, 35 and 20 kDa have been identified in activated rat HSCs, with the 35-kDa isoform being predominant[44,45]. Attard et al[44] noted that activation of the α1 (I) collagen promoter by acetaldehyde in HSCs is most likely consequent upon an increase in this isoform and increased protein/DNA binding to the C/EBP binding site.
Another report suggests that acetaldehyde-induced α1 (I) gene expression in rat HSCs requires the binding of the acetaldehyde-inducible transcription factor BTEB to a GC box (-1484 to -1476) on the promoter of this gene[43]. In keeping with this proposal, blocking BTEB protein production, results in a reduction in acetaldehyde-induced α1 (I) collagen gene expression[43]. In an extension of the previous report, additional data suggest that acetaldehyde can firstly induce AP-1 activation in HSCs[38,43] and then the activated AP-1 can bind to AP-1 responsive elements in the BTEB promoter to stimulate BTEB expression. The BTEB protein, in turn, stimulates the expression of the α1 (I) gene in HSCs[38,43].
Nuclear factor I (NF-I), a CCAAT binding trans-cription factor, is also known to bind to and activate the α1 (I) and α2 (I) collagen promoters[46]. Acetaldehyde-induced enhancement of the α2 (I) collagen promoter in activated HSCs is associated with increased binding of NF-I to a consensus consequence located at -352 to -104 bp from the transcriptional start site[34,46,47].
These data suggest that the transcription factors C/EBP, BTEB and NF-1 bind to and activate type I collagen gene transcription through each of them and/or via synergic effects, though further characterization of these effects is required. Whether these collagen gene transcription signaling pathways (after exposure to ethanol), are regulated by acetaldehyde itself, or in concert with other profibrogenic mediators such as oxidative stress or TGFβ1 is presently uncertain. The available data are discussed below.
Oxidative stress: Increased oxidative stress is present in the liver after both acute and chronic ethanol administration[48]. Ethanol-induced oxidative stress within hepatocytes can occur acutely through ethanol metabolism or chronically following the induction of CYP2E1[2,10]. The oxidative metabolism of ethanol in hepatocytes elicits a range of mediators including ROS. CYP2E1 in particular has been shown to generate ROS including the superoxide anion, hydrogen peroxide (H2O2) and hydroxyethyl free radicals[2,49]. Other sources of free radical generation by ethanol include NADH oxidation by aldehyde oxidase[50].
HSCs contain the enzymes of oxidative ethanol metabolism including ADH and P450 proteins[51-53]. Yamada and Oinonen[52] observed that CYP2E1 is present in rat HSCs as high as 21% of that found in hepatocytes. CYP2E1 is also detectable in the rat hepatic stellate cell line, HSC-T6[51]. In HSC-T6 cells overexpressing ethanol-inducible CYP2E1, time- and dose-dependent induction in collagen α2 (I) mRNA together with increased H2O2 production by ethanol has been observed. Antioxidants, including catalase (an H2O2 scavenger) prevent this increase in collagen α2 (I) mRNA expression[51]. Because ethanol can be oxidized to acetaldehyde by the peroxidative activity of catalase[54], this decrease in collagen α2 (I) expression by catalase suggests that ethanol-derived acetaldehyde is not responsible for this effect. Svegliati-Baroni et al[11] and Greenwel et al[35] have also provided evidence to support the concept that increases in mouse α1 (I) and human α2 (I) collagen gene expression in HSCs by acetaldehyde are linked to elevated H2O2 production. For example, acetaldehyde-elicited type I collagen gene expression can be blocked by the addition of catalase[11,35], and is in part, TGFβ1-independent[11]. It is known that H2O2 activates MAPK pathways[49] and this activity might enhance the binding of the down stream transcription factors to acetaldehyde-responsive elements within the type I collagen promoter. Likewise, leptin induces H2O2 production and contributes to TIMP-1 expression in HSCs[55]. Collectively, these data suggest that increased H2O2 generation during the metabolism of ethanol by HSCs might play a critical role in their activation.
TGFβ-1: Ethanol and acetaldehyde increase autocrine TGFβ1 expression in HSCs. In turn, TGFβ1 is able to up-regulate type I collagen gene expression[32,34,38]. Anania and colleagues[34] noted that the effects of acetaldehyde-induced TGFβ1 in the regulation of α2 (I) collagen gene expression are mediated by a factor or factors that bind to nuclear factor I (NF-I) consensus sequence located at the -352 to -104 region of the α2 (I) gene promoter. Acetaldehyde further increases the secretion of both latent and active forms of TGFβ1 in cultured rat HSCs[38], and induces the expression of the type II TGFβ receptor which is required for all TGFβ-mediated signaling events[38]. In transient transfection experiments, the combination of TGFβ1 and acetaldehyde could result in greater activation of the mouse α2 (I) collagen promoter than either TGFβ1 or acetaldehyde alone[34]. Taken together, these observations suggest that TGFβ1 could play a key role in acetaldehyde-induced collagen I gene activation.
Chen et al[38,43] have noted that acetaldehyde stimulates latent TGFβ1 secretion and TGFβ type II receptor gene expression. BTEB might be the principal transcription factor binding to the GC box of the type II TGFβ receptor gene promoter[38]. The authors proposed a model wherein acetaldehyde activates signal transduction pathways including PKC, JNK and ERK, leading to activation of AP-1. AP-1 is proposed to activate the gene expression of BTEB. BTEB then up-regulates TGFβ type II receptor gene expression in HSCs. By stimulating latent TGFβ1 activation and secretion, as well as up-regulating the expression of TGFβ type II receptor, acetaldehyde activates TGFβ1 signaling, which eventually enhances expression of the α1 (I) collagen gene in HSCs[38].
The precise molecular mechanisms by which acetaldehyde elicits TGFβ1 production in HSCs are largely unknown. Acetaldehyde might directly bind to the TGFβ1 gene promoter leading to its activation. Alternatively, acetaldehyde might bind to other gene promoters of transcription factors that in turn activate the TGFβ1 gene.
Rodriguez-Fragoso and his colleagues[56] investigated the effects of the activity of urokinase type plasminogen activator (uPA) in the CFSC-2G stellate cell line and demonstrated that acetaldehyde (175, 250 and 350 μmol/L) enhances uPA gene expression. This is accompanied with a concomitant increase in production of type I collagen. uPA plays an important role in matrix remodeling under a wide range of physiological and pathological conditions, activates TGFβ1 and induces proliferation of HSCs[57,58]. Furthermore, profibrogenic mediators including IL-6, TNF-α, malondialdehyde (MDA) and intracellular GSSG have been reported to increase in CFSC-2G cells treated with ethanol or acetaldehyde[59-61].
Malondialdehyde-acetaldehyde (MAA) -protein adducts induce a dose- and time-dependent increase in the secretion of chemokines including monocyte chemoattractant protein (MCP)-1 and macrophage inflammatory protein (MIP)-2 as well as an increase in the production and expression of intercellular adhesion molecule-1 (ICAM-1) in activated rat HSCs[62,63]. These effects may contribute further to the activation of HSCs and the subsequent development of alcohol-associated liver fibrosis.
Rat pancreatic stellate cells (PSCs) exhibit features similar to those of HSCs[64-66]. These cells are abundant in alcoholic chronic pancreatitis in humans, suggesting a central role of this cell type in pancreatic fibrosis[65]. An effect of ethanol on the modulation of PSCs has been documented. Ethanol and acetaldehyde increase α-SMA protein and type I collagen synthesis in PSCs[67], likewise enhance PSC MMP-2 and TIMP-2 gene expression as well as TIMP-2 protein secretion[67-69]. Both ethanol and acetaldehyde increase the activation of all 3 subfamilies (ERK1/2, JNK/SAPK and p38 kinase) of the MAPK pathway in PSCs. Only p38 MAPK is responsible however, for the induction of α-SMA and α1 (I) collagen gene expression[70]. Moreover, ethanol and acetaldehyde-induced MAPK activation can be blocked by the antioxidant N-acetyl-cysteine, suggesting a role of oxidative stress in signal transduction[68,71].
Ethanol can be metabolized in hepatocytes and stellate cells to generate acetaldehyde and other metabolites. Ethanol and/or its metabolites including acetaldehyde have direct effects on HSC activation. These effects might be mediated by ethanol/acetaldehyde and/or ethanol/acetaldehyde-induced oxidative stress and TGFβ1 expression which activate relevant signaling pathways leading to the binding of transcription factors to the type I collagen gene promoter (Figure 2). As a result, ethanol augments the production of extracellular matrix proteins. Ethanol also stimulates the production of other profibrotic mediators, including IL-6, TNF-α and uPA. Taken together, these effects of ethanol/acetaldehyde on HSCs play an important role in the development of alcohol-associated liver fibrosis. Characterization of the key genes initiating and perpetuating the process of HSC activation by ethanol helps to further elucidate the molecular mechanisms of alcohol-associated liver fibrosis. In the future, it is hoped that specific, directed pharmacological agents can be selected and/or developed that target these mechanisms and thereby prevent or retard the fibrogenesis induced by alcohol.
S- Editor Wang J L- Editor Wang XL E- Editor Ma WH
| 1. | Lieber CS. Metabolism of alcohol. Clin Liver Dis. 2005;9:1-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 169] [Article Influence: 8.5] [Reference Citation Analysis (2)] |
| 2. | Lieber CS. Alcoholic fatty liver: its pathogenesis and mechanism of progression to inflammation and fibrosis. Alcohol. 2004;34:9-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 424] [Cited by in RCA: 450] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 3. | Schuppan D, Atkinson J, Ruehl M, Riecken EO. Alcohol and liver fibrosis--pathobiochemistry and treatment. Z Gastroenterol. 1995;33:546-550. [PubMed] |
| 4. | Lieber CS. Alcoholic liver injury: pathogenesis and therapy in 2001. Pathol Biol (Paris). 2001;49:738-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Lieber CS. Ethanol metabolism, cirrhosis and alcoholism. Clin Chim Acta. 1997;257:59-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 258] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 6. | Eriksson CJ. The role of acetaldehyde in the actions of alcohol (update 2000). Alcohol Clin Exp Res. 2001;25:15S-32S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 169] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 7. | Arteel GE. Oxidants and antioxidants in alcohol-induced liver disease. Gastroenterology. 2003;124:778-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 362] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 8. | Greenwel P. Acetaldehyde-mediated collagen regulation in hepatic stellate cells. Alcohol Clin Exp Res. 1999;23:930-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Friedman SL. Stellate cell activation in alcoholic fibrosis--an overview. Alcohol Clin Exp Res. 1999;23:904-910. [PubMed] |
| 10. | Nieto N, Friedman SL, Cederbaum AI. Stimulation and proliferation of primary rat hepatic stellate cells by cytochrome P450 2E1-derived reactive oxygen species. Hepatology. 2002;35:62-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 200] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 11. | Svegliati-Baroni G, Inagaki Y, Rincon-Sanchez AR, Else C, Saccomanno S, Benedetti A, Ramirez F, Rojkind M. Early response of alpha2(I) collagen to acetaldehyde in human hepatic stellate cells is TGF-beta independent. Hepatology. 2005;42:343-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 70] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 12. | Matsuoka M, Zhang MY, Tsukamoto H. Sensitization of hepatic lipocytes by high-fat diet to stimulatory effects of Kupffer cell-derived factors: implication in alcoholic liver fibrogenesis. Hepatology. 1990;11:173-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Tsukamoto H, Cheng S, Blaner WS. Effects of dietary polyunsaturated fat on ethanol-induced Ito cell activation. Am J Physiol. 1996;270:G581-G586. [PubMed] |
| 14. | Hendriks HF, Verhoofstad WA, Brouwer A, de Leeuw AM, Knook DL. Perisinusoidal fat-storing cells are the main vitamin A storage sites in rat liver. Exp Cell Res. 1985;160:138-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 181] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 15. | Chambon P. A decade of molecular biology of retinoic acid receptors. FASEB J. 1996;10:940-954. [PubMed] |
| 16. | Weiner FR, Blaner WS, Czaja MJ, Shah A, Geerts A. Ito cell expression of a nuclear retinoic acid receptor. Hepatology. 1992;15:336-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 38] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Leo MA, Lieber CS. Alcohol, vitamin A, and beta-carotene: adverse interactions, including hepatotoxicity and carcinogenicity. Am J Clin Nutr. 1999;69:1071-1085. [PubMed] |
| 18. | Wang XD. Chronic alcohol intake interferes with retinoid metabolism and signaling. Nutr Rev. 1999;57:51-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Sauvant P, Sapin V, Abergel A, Schmidt CK, Blanchon L, Alexandre-Gouabau MC, Rosenbaum J, Bommelaer G, Rock E, Dastugue B. PAV-1, a new rat hepatic stellate cell line converts retinol into retinoic acid, a process altered by ethanol. Int J Biochem Cell Biol. 2002;34:1017-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Cottalasso D, Bassi AM, Canepa C, Maloberti G, Casu A, Nanni G. Chronic ethanol treatment: dolichol and retinol distribution in isolated rat liver cells. Free Radic Biol Med. 2003;34:337-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Wang L, Attard FA, Tankersley LR, Potter JJ, Mezey E. Effect of retinoic acid on the enhancing effect of acetaldehyde on mouse type I collagen expression. Arch Biochem Biophys. 2000;376:191-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Li J, Hu W, Baldassare JJ, Bora PS, Chen S, Poulos JE, O'Neill R, Britton RS, Bacon BR. The ethanol metabolite, linolenic acid ethyl ester, stimulates mitogen-activated protein kinase and cyclin signaling in hepatic stellate cells. Life Sci. 2003;73:1083-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Gressner AM, Althaus M. Effects of ethanol, acetaldehyde, and lactate on proteoglycan synthesis and proliferation of cultured rat liver fat-storing cells. Gastroenterology. 1988;94:797-807. [PubMed] |
| 24. | Poniachik J, Baraona E, Zhao J, Lieber CS. Dilinoleoylphosphatidylcholine decreases hepatic stellate cell activation. J Lab Clin Med. 1999;133:342-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Oide H, Itatsu T, Hirose M, Wang XE, Nishiyama D, Takei Y, Sato N. Acute and chronic effect of alcohol on Ca2+ channels in hepatic stellate cells. Alcohol Clin Exp Res. 2000;24:357-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 26. | Itatsu T, Oide H, Watanabe S, Tateyama M, Ochi R, Sato N. Alcohol stimulates the expression of L-type voltage-operated Ca2+ channels in hepatic stellate cells. Biochem Biophys Res Commun. 1998;251:533-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Chen A, Zhang L, Xu J, Tang J. The antioxidant (-)-epigallocatechin-3-gallate inhibits activated hepatic stellate cell growth and suppresses acetaldehyde-induced gene expression. Biochem J. 2002;368:695-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 70] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Benyon RC, Arthur MJ. Extracellular matrix degradation and the role of hepatic stellate cells. Semin Liver Dis. 2001;21:373-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 370] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 29. | Fontana L, Jerez D, Rojas-Valencia L, Solís-Herruzo JA, Greenwel P, Rojkind M. Ethanol induces the expression of alpha 1(I) procollagen mRNA in a co-culture system containing a liver stellate cell-line and freshly isolated hepatocytes. Biochim Biophys Acta. 1997;1362:135-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 30. | Moshage H, Casini A, Lieber CS. Acetaldehyde selectively stimulates collagen production in cultured rat liver fat-storing cells but not in hepatocytes. Hepatology. 1990;12:511-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 180] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 31. | Li J, Kim CI, Leo MA, Mak KM, Rojkind M, Lieber CS. Polyunsaturated lecithin prevents acetaldehyde-mediated hepatic collagen accumulation by stimulating collagenase activity in cultured lipocytes. Hepatology. 1992;15:373-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 84] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 32. | Casini A, Cunningham M, Rojkind M, Lieber CS. Acetaldehyde increases procollagen type I and fibronectin gene transcription in cultured rat fat-storing cells through a protein synthesis-dependent mechanism. Hepatology. 1991;13:758-765. [PubMed] |
| 33. | Greenwel P, Domínguez-Rosales JA, Mavi G, Rivas-Estilla AM, Rojkind M. Hydrogen peroxide: a link between acetaldehyde-elicited alpha1(I) collagen gene up-regulation and oxidative stress in mouse hepatic stellate cells. Hepatology. 2000;31:109-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 155] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 34. | Anania FA, Potter JJ, Rennie-Tankersley L, Mezey E. Activation by acetaldehyde of the promoter of the mouse alpha2(I) collagen gene when transfected into rat activated stellate cells. Arch Biochem Biophys. 1996;331:187-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 35. | Svegliati-Baroni G, Ridolfi F, Di Sario A, Saccomanno S, Bendia E, Benedetti A, Greenwel P. Intracellular signaling pathways involved in acetaldehyde-induced collagen and fibronectin gene expression in human hepatic stellate cells. Hepatology. 2001;33:1130-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 112] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 36. | Casini A, Ceni E, Salzano R, Milani S, Schuppan D, Surrenti C. Acetaldehyde regulates the gene expression of matrix-metalloproteinase-1 and -2 in human fat-storing cells. Life Sci. 1994;55:1311-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 37. | Anania FA, Womack L, Jiang M, Saxena NK. Aldehydes potentiate alpha(2)(I) collagen gene activity by JNK in hepatic stellate cells. Free Radic Biol Med. 2001;30:846-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 38. | Chen A. Acetaldehyde stimulates the activation of latent transforming growth factor-beta1 and induces expression of the type II receptor of the cytokine in rat cultured hepatic stellate cells. Biochem J. 2002;368:683-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 69] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 39. | Anania FA, Womack L, Potter JJ, Mezey E. Acetaldehyde enhances murine alpha2(I) collagen promoter activity by Ca2+-independent protein kinase C activation in cultured rat hepatic stellate cells. Alcohol Clin Exp Res. 1999;23:279-284. [PubMed] |
| 40. | Casini A, Galli G, Salzano R, Ceni E, Franceschelli F, Rotella CM, Surrenti C. Acetaldehyde induces c-fos and c-jun proto-oncogenes in fat-storing cell cultures through protein kinase C activation. Alcohol Alcohol. 1994;29:303-314. [PubMed] |
| 41. | Mann DA, Smart DE. Transcriptional regulation of hepatic stellate cell activation. Gut. 2002;50:891-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 155] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 42. | Eng FJ, Friedman SL. Transcriptional regulation in hepatic stellate cells. Semin Liver Dis. 2001;21:385-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 43. | Chen A, Davis BH. The DNA binding protein BTEB mediates acetaldehyde-induced, jun N-terminal kinase-dependent alphaI(I) collagen gene expression in rat hepatic stellate cells. Mol Cell Biol. 2000;20:2818-2826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 70] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 44. | Attard FA, Wang L, Potter JJ, Rennie-Tankersley L, Mezey E. CCAAT/enhancer binding protein beta mediates the activation of the murine alpha1(I) collagen promoter by acetaldehyde. Arch Biochem Biophys. 2000;378:57-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 45. | Attard FA, Wang L, Potter JJ, Rennie-Tankersley L, Mezey E. Identification of new sites of binding and activation of the murine alpha1(I) collagen promoter by CCAAT/enhancer binding protein beta. DNA Cell Biol. 2001;20:455-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 46. | Anania FA, Potter JJ, Rennie-Tankersley L, Mezey E. Effects of acetaldehyde on nuclear protein binding to the nuclear factor I consensus sequence in the alpha 2(I) collagen promoter. Hepatology. 1995;21:1640-1648. [PubMed] |
| 47. | Miao K, Potter JJ, Anania FA, Rennie-Tankersley L, Mezey E. Effect of acetaldehyde on Sp1 binding and activation of the mouse alpha 2(I) collagen promoter. Arch Biochem Biophys. 1997;341:140-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 48. | Cahill A, Cunningham CC, Adachi M, Ishii H, Bailey SM, Fromenty B, Davies A. Effects of alcohol and oxidative stress on liver pathology: the role of the mitochondrion. Alcohol Clin Exp Res. 2002;26:907-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 107] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 49. | Aroor AR, Shukla SD. MAP kinase signaling in diverse effects of ethanol. Life Sci. 2004;74:2339-2364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 170] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 50. | Kono H, Rusyn I, Bradford BU, Connor HD, Mason RP, Thurman RG. Allopurinol prevents early alcohol-induced liver injury in rats. J Pharmacol Exp Ther. 2000;293:296-303. [PubMed] |
| 51. | Nieto N, Greenwel P, Friedman SL, Zhang F, Dannenberg AJ, Cederbaum AI. Ethanol and arachidonic acid increase alpha 2(I) collagen expression in rat hepatic stellate cells overexpressing cytochrome P450 2E1. Role of H2O2 and cyclooxygenase-2. J Biol Chem. 2000;275:20136-20145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 98] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 52. | Yamada T, Imaoka S, Kawada N, Seki S, Kuroki T, Kobayashi K, Monna T, Funae Y. Expression of cytochrome P450 isoforms in rat hepatic stellate cells. Life Sci. 1997;61:171-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 53. | Yamauchi M, Potter JJ, Mezey E. Characteristics of alcohol dehydrogenase in fat-storing (Ito) cells of rat liver. Gastroenterology. 1988;94:163-169. [PubMed] |
| 54. | Thurman RG. Induction of hepatic microsomal reduced nicotinamide adenine dinucleotide phosphate-dependent production of hydrogen peroxide by chronic prior treatment with ethanol. Mol Pharmacol. 1973;9:670-675. [PubMed] |
| 55. | Cao Q, Mak KM, Ren C, Lieber CS. Leptin stimulates tissue inhibitor of metalloproteinase-1 in human hepatic stellate cells: respective roles of the JAK/STAT and JAK-mediated H2O2-dependant MAPK pathways. J Biol Chem. 2004;279:4292-4304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 138] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 56. | Rodríguez-Fragoso L, Alvarez R, Reyes-Esparza JA, Garcés ME. Acetaldehyde increases the activity and gene expression of urokinase type plasminogen activator in a hepatic stellate cell line. Toxicology. 1999;137:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 57. | Kharbanda KK, Shubert KA, Wyatt TA, Sorrell MF, Tuma DJ. Effect of malondialdehyde-acetaldehyde-protein adducts on the protein kinase C-dependent secretion of urokinase-type plasminogen activator in hepatic stellate cells. Biochem Pharmacol. 2002;63:553-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 207] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 58. | Rieder H, Armbrust T, Meyer zum Büschenfelde KH, Ramadori G. Contribution of sinusoidal endothelial liver cells to liver fibrosis: expression of transforming growth factor-beta 1 receptors and modulation of plasmin-generating enzymes by transforming growth factor-beta 1. Hepatology. 1993;18:937-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 59. | Hernández E, Correa A, Bucio L, Souza V, Kershenobich D, Gutiérrez-Ruiz MC. Pentoxifylline diminished acetaldehyde-induced collagen production in hepatic stellate cells by decreasing interleukin-6 expression. Pharmacol Res. 2002;46:435-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 60. | Gutiérrez-Ruiz MC, Bucio L, Correa A, Souza V, Hernández E, Gómez-Quiroz LE, Kershenobich D. Metadoxine prevents damage produced by ethanol and acetaldehyde in hepatocyte and hepatic stellate cells in culture. Pharmacol Res. 2001;44:431-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 61. | Quiroz SC, Bucio L, Souza V, Hernández E, González E, Gómez-Quiroz L, Kershenobich D, Vargas-Vorackova F, Gutiérrez-Ruiz MC. Effect of endotoxin pretreatment on hepatic stellate cell response to ethanol and acetaldehyde. J Gastroenterol Hepatol. 2001;16:1267-1273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 62. | Kharbanda KK, Todero SL, Shubert KA, Sorrell MF, Tuma DJ. Malondialdehyde-acetaldehyde-protein adducts increase secretion of chemokines by rat hepatic stellate cells. Alcohol. 2001;25:123-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 63. | Kharbanda KK, Todero SL, Sorrell MF, Tuma DJ. MAA adducts increase chemokine production and the expression of cell adhesion molecule, ICAM-1, in hepatic stellate cells. Hepatology. 1998;28:311A. |
| 64. | Apte MV, Haber PS, Applegate TL, Norton ID, McCaughan GW, Korsten MA, Pirola RC, Wilson JS. Periacinar stellate shaped cells in rat pancreas: identification, isolation, and culture. Gut. 1998;43:128-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 719] [Cited by in RCA: 716] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 65. | Bachem MG, Schneider E, Gross H, Weidenbach H, Schmid RM, Menke A, Siech M, Beger H, Grünert A, Adler G. Identification, culture, and characterization of pancreatic stellate cells in rats and humans. Gastroenterology. 1998;115:421-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 744] [Cited by in RCA: 798] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 66. | Haber PS, Keogh GW, Apte MV, Moran CS, Stewart NL, Crawford DH, Pirola RC, McCaughan GW, Ramm GA, Wilson JS. Activation of pancreatic stellate cells in human and experimental pancreatic fibrosis. Am J Pathol. 1999;155:1087-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 323] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 67. | Apte MV, Phillips PA, Fahmy RG, Darby SJ, Rodgers SC, McCaughan GW, Korsten MA, Pirola RC, Naidoo D, Wilson JS. Does alcohol directly stimulate pancreatic fibrogenesis Studies with rat pancreatic stellate cells. Gastroenterology. 2000;118:780-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 182] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 68. | Masamune A, Kikuta K, Satoh M, Satoh A, Shimosegawa T. Alcohol activates activator protein-1 and mitogen-activated protein kinases in rat pancreatic stellate cells. J Pharmacol Exp Ther. 2002;302:36-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 85] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 69. | Phillips PA, McCarroll JA, Park S, Wu MJ, Pirola R, Korsten M, Wilson JS, Apte MV. Rat pancreatic stellate cells secrete matrix metalloproteinases: implications for extracellular matrix turnover. Gut. 2003;52:275-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 217] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 70. | McCarroll JA, Phillips PA, Park S, Doherty E, Pirola RC, Wilson JS, Apte MV. Pancreatic stellate cell activation by ethanol and acetaldehyde: is it mediated by the mitogen-activated protein kinase signaling pathway. Pancreas. 2003;27:150-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 63] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 71. | Purohit V, Brenner DA. Mechanisms of alcohol-induced hepatic fibrosis: a summary of the Ron Thurman Symposium. Hepatology. 2006;43:872-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 130] [Article Influence: 6.8] [Reference Citation Analysis (0)] |