Published online Nov 14, 2006. doi: 10.3748/wjg.v12.i42.6766
Revised: August 5, 2006
Accepted: August 13, 2006
Published online: November 14, 2006
AIM: To evaluate the growth inhibition efficacy of atofluding derivative N3-o-toluyl-fluorouracil (TFU) on human gastric carcinoma cell lines SGC-7901 and MKN-45.
METHODS: Cell growth inhibition by TFU was measured by MTT and clonogenic assays without or with liver microsomal enzymes. Xenografts of cancer cells in nude mice were employed to study the anti-proliferative effects of TFU in vivo.
RESULTS: TFU inhibited the growth of SGC-7901 and MKN-45 cells. However, the inhibitory effects of TFU on cell growth were not significant. The inhibition rates were enhanced in the presence of liver microsomal enzymes, ranging 4.73%-48.57% in SGC-7901 cells and 9.0%-62.02% in MKN-45 cells. In vivo, TFU delayed the growth of SGC-7901 and MKN-45 cells in nude mice. The inhibition rates were 40.49%, 63.24%, and 75.98% in SGC-7901 cells and 40.76%, 61.41%, and 82.07% in MKN-45 cells when the oral doses were 25, 50, and 100 mg/kg, respectively. TFU treatment was generally well tolerated by mice with less than 20% reduction in body weight.
CONCLUSION: TFU inhibits the growth of human gastric carcinoma cells. The inhibition rates are increased in the presence of liver microsomal enzymes. The efficacy of TFU may be associated with the sustaining release of 5-fluorouracil (5-FU) mediated by the enzymes.
- Citation: Liu J, Xu WF, Cui SX, Zhou Y, Yuan YX, Chen MH, Wang RH, Gai RY, Makuuchi M, Tang W, Qu XJ. Inhibition of human gastric carcinoma cell growth by atofluding derivative N3-o-toluyl-fluorouracil. World J Gastroenterol 2006; 12(42): 6766-6770
- URL: https://www.wjgnet.com/1007-9327/full/v12/i42/6766.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i42.6766
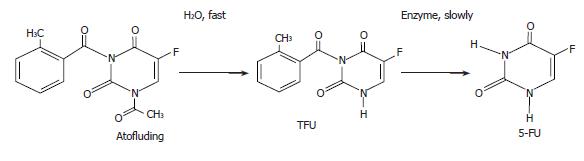
5-fluorouracil (5-FU), a pyrimidine analog, has become a backbone in the therapy of gastric and colon cancer since its introduction in 1957. However, its clinically effective dosage is very close to its toxic dosage when given intravenously, resulting in strong toxicities to gastric and intestinal mucosa and bone marrow[1-3]. In addition, its plasma half-life is very short (15-20 min) and the drug is administered only as a continuous iv infusion[4-7]. In order to overcome these disadvantages, attempts have been made to design and synthesize new 5-FU derivatives. In the 1980s, a new oral 5-FU derivative, N1-acetyl-N3-o-toluyl-fluorouracil (atofluding), was developed[8]. Studies have shown that atofluding can effectively treat many types of tumor with few side effects. Nevertheless, the acetyl group on the N1 position of atofluding is not stable and prone to decompose, impairing quality control for the preparation. The pharmacokinetics showed that atofluding, N3-o-toluyl-fluorouracil (TFU), rather than atofluding itself could be detected in the serum after oral administration. TFU is the decomposition products of atofluding that hydrolyze the acetyl group by fluorine on C5 position (Figure 1). TFU is extracted and dissolved in acetone and water before its determination by high-pressure liquid chromatography[8,9]. The results also showed that steady-state concentrations of TFU can remain in the body for 30 h[8]. These results have led us to the conclusion that TFU is stable in the preparation. Encouraged by these observations, the anti-tumor activities of TFU were studied and the inhibition efficacy of TFU on the growth of human gastric carcinoma cells was evaluated in the present study in order to replace the unstable atofluding.
Human gastric carcinoma cell lines, SGC-7901 and MKN-45 were obtained from the Division of Cancer Treatment, National Cancer Institute of China (Beijing, China). Cells were maintained in RPMI-1640 supplemented with 10% (v/v) heat-inactivated fetal bovine serum, penicillin-streptomycin (100 IU/mL-100 µg/mL), 2 mmol/L glutamine, and 10 mM Hepes buffer at 37°C in a humid atmosphere containing 50 mL/L CO2. Cells were fed every three to four days, and harvested by brief incubation in PBS containing 0.02% trypsin-EDTA.
TFU was synthesized by acylation of 5-FU with 2-methylbenzoyl chloride in pyridine at room temperature as previously described[8,9] and dissolved in dimethylsulfoxide (DMSO) for in vitro assay and in 5% amylum for in vivo study.
Cells (2-5 × 104 per well) seeded in 96-well plates (Corning Costar Corporation, Cambridge, MA, USA) for 12 h were treated with different doses of TFU for the required time period. The medium was then removed and the wells were washed with PBS. Cell viability was assessed by adding 20 µL of MTT [3-(4, 5-dimethylthiazol-2-yl)-2], 5 mg/mL 5-diphenyltetrazolium bromide (Sigma, USA) for 4 h[10]. Light absorbance of the solution was measured at 540 nm on the plate reader (TECAN, Grodig, Salzburg, Austria). The growth of experimental and control cells was compared. Experiments were performed in triplicate.
SD male rats (150 ± 10 g) from the animal breeding house were treated with intra-peritoneal injections of phenobarbital (80 mg/kg body weight) daily for three consecutive days in 0.5 mL of peanut oil. The animals were sacrificed 24 h after the last injection. Livers were removed, washed with chilled 0.1 mol/L phosphate buffer (pH 7.4) and homogenized. Liver microsomal enzymes were prepared as described by Rastogi S et al[11]. The microsomal enzymes were resuspended in 0.1 mol/L phosphate buffer (pH 7.4) containing 10 mmol/L dithiothrietol, 10 mmol/L EDTA and 20% glycerol. Ten mL of the mixture of enzymes containing 1 mL of microsomes and 31.4 mg of nicotinamide adenine dinucleotide phosphate (NADPH; Merck, Darmstadt, Germany) was used[12]. The enzyme mixture (2 μL) was applied to each well of the 96-well plates after cells were seeded and TFU was added. Cell growth inhibition was measured as above.
Cells (250 to 300 per well) grown in 6-well plates (Falcon, Becton Dickinson, Franklin lakes, New Jersey, USA) for 12 h were treated with different doses of TFU at 37°C. After two weeks, colonies (greater than 50 cells) were stained with crystal violet and counted as previously described[10,13].
The in vivo efficacy of TFU was assessed in nude mice bearing tumors. Balb/c athymic (nu+/nu+) female mice, 4-6 wk of age, were purchased from the Experimental Animal Laboratory, Chinese Academy of Medical Sciences (Beijing, China). The research protocol was approved in accordance with the institutional guidelines of the Animal Care and Use Committee at Shandong University. Animals were housed under pathogen-free conditions. Cells (1 × 107) were suspended in 100 µL of Matrigel (Collaborative Biomedical, Bedford, MA, USA) and injected subcutaneously into the right anterior flank of nude mice. After seven days, when tumor volume reached approximately 0.1-0.2 cm3, the mice were divided into different groups (n = 8) and orally administered 0, 25, 50, 100 mg/kg of TFU in 0.5 mL of 5% amylum[14]. Administrations were performed six days per week for three consecutive weeks. Tumor growth inhibition rates were defined as a ratio to the control tumor weight.
Statistical significance was determined by the Student’s two-tailed t-test. The Kruskal-Wallis test was used to detect the percent of cell growth inhibition. P < 0.05 was considered statistically significant. Statistical analysis was performed with SPSS/Win11.0 software (SPSS, Chicago, IL, USA).
To select optimal times for analysis of the relationship between treatment with TFU and cell growth inhibition, untreated cells were tested over six days. SGC-7901 and MKN-45 cells reached their confluence by d 6. We then selected d 5 within the linear growth period for concentration analysis and a 5-d period for the time course of treated cells (data not shown).
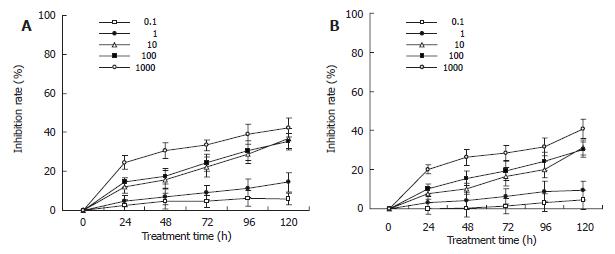
Gastric carcinoma cells were treated with TFU (0.1, 1, 10, 100, 1000 μg/mL) for up to 120 h and the viable cells were evaluated as described in MATERIALS and METHODS. As shown in Figure 2A, TFU showed its anti-proliferative effects on SGC-7901 cells in a dose- and time-dependent manner. Significant differences were seen across the doses tested (Kruskal-wallis H = 18.870, P = 0.042). The growth inhibitory effects of TFU were significantly increased from 24 to 120 h of incubation (Kruskal-wallis H = 18.870, P = 0.042). However, the inhibitory effects of TFU on cell growth were not high. Cells cultured with 10 µg/mL of TFU achieved only 11.09% of growth inhibition at 24 h, 15.69% at 48 h, 22.24% at 72 h, 28.93% at 96 h and 36.86% at 120 h. The maximum inhibition rate was 42.51% at the concentration of 1000 µg/mL after 120 h treatment. MKN-45 cells expressed similar results (Figure 2B).
The inhibitory effects of TFU on human gastric carcinoma cells were then examined in the presence of liver microsomal enzymes. The inhibition rates were significantly increased at all concentrations (0.1, 1, 10, 100, 1000 µg/mL) and treatment time points (24 to 120 h), being 4.73%-48.57% in SGC-7901 cells and 9.0%-62.02% in MKN-45 cells (Table 1). The growth inhibition was increased in a dose- and time-dependent manner (data not shown).
| Cell line | Time (h) | Inhibition (%) | ||||
| 0.1 μg/mL | 1 μg/mL | 10 μg/mL | 100 μg/mL | 1000 μg/mL | ||
| SGC-7901 | ||||||
| 24 | 4.73 | 8.21 | 11.92 | 14.84 | 24.08 | |
| 48 | 9.56 | 15.10 | 22.31 | 36.02 | 39.89 | |
| 72 | 17.29 | 24.35 | 30.75 | 40.03 | 43.72 | |
| 96 | 22.03 | 31.76 | 32.97 | 42.43 | 45.51 | |
| 120 | 37.67 | 36.67 | 35.24 | 45.86 | 48.57 | |
| MKN-45 | ||||||
| 24 | 9.00 | 18.38 | 20.00 | 36.99 | 42.02 | |
| 48 | 20.00 | 34.00 | 40.00 | 51.40 | 55.00 | |
| 72 | 45.09 | 52.63 | 57.11 | 58.18 | 60.16 | |
| 96 | 52.50 | 61.64 | 57.87 | 55.53 | 61.41 | |
| 120 | 59.19 | 62.02 | 59.12 | 55.84 | 53.98 | |
We also used a clonogenic assay to further test the sensitivity of SGC-7901 and MKN-45 cells to TFU over a relatively long culture period (two weeks). The anti-proliferative effect of TFU on clone formation was significantly increased in the presence of liver microsomal enzymes across the concentrations (P < 0.01, data not shown).
The effects of TFU on gastric carcinoma cell xenografts in nude mice were then examined. As shown in Table 2, TFU delayed the growth of SGC-7901 and MKN-45 cells after three weeks of treatment with 25, 50, and 100 mg/kg (P < 0.01). TFU treatment inhibited tumor growth in a dose-dependent manner. This inhibitory effect on the xenograft was more pronounced in MKN-45 cells than in SGC-7901 cells. Except for the 100 mg/kg group, TFU treatment was generally well tolerated by mice with less than 20% reduction in body weight (P > 0.05).
| Cell line | Dosage(mg/kg) | Body weight(mean ± SD, g) | Tumor weight(mean ± SD, g) | Tumor growthinhibition (%) |
| SGC-7901 | ||||
| 0 | 22.12 ± 2.31 | 2.30 ± 0.67 | ||
| 25 | 21.62 ± 1.96 | 1.21 ± 0.61 | 30.49 | |
| 50 | 20.28 ± 2.95 | 0.75 ± 0.17 | 53.24 | |
| 100 | 18.05 ± 2.40 | 1.23 ± 0.43 | 65.98 | |
| MKN-45 | ||||
| 0 | 22.72 ± 1.75 | 1.84 ± 0.72 | ||
| 25 | 21.62 ± 1.96 | 1.09 ± 0.81 | 40.76 | |
| 50 | 20.28 ± 2.95 | 0.71 ± 0.40 | 61.41 | |
| 100 | 18.75 ± 1.44 | 0.33 ± 0.49 | 82.07 | |
In this report, we evaluated the efficacy of TFU, the pro-drug of 5-FU, on growth inhibition of human gastric carcinoma cells. The results showed that TFU had anti-proliferative effects on SGC-7901 and MKN-45 cell growth. The inhibition rates were increased significantly in the presence of liver microsomal enzymes.
Structurally, the difference between atofluding and TFU is that the former has an acetyl group on its N1 position. Nevertheless, the acetyl group is prone to be hydrolyzed into TFU rapidly by fluorine on the C5 position, impairing quality control for the preparation. On the other hand, TFU is very stable in vitro and in vivo[8,9,15]. Consistent results have been seen in our previous studies. The pharmacokinetic studies showed that TFU is detectable some minutes after oral administration of atofluding[8]. In this study, however, atofluding was not detected in the required time period, the steady-state concentrations of TFU in blood (9.97 ± 0.7 mg/mL) were achieved 30 h after multiple administrations. TFU was subsequently slowly metabolized to release 5-FU. Though the subsequent steady-state concentrations of 5-FU were lower than those of TFU, the concentrations could remain for up to 50-52 h[15], suggesting that TFU has good anti-tumor activities and low side effects. TFU would replace the unstable atofluding as the preparation. Sustained release of 5-FU from TFU is a catalytic procedure and enzymes are needed to mediate the bio-transformation[8]. In this study, liver microsomal enzymes promoted the activities of TFU, suggesting that the enzymes can mediate the metabolism.
However, the mechanism of the pathways is not known. Liver microsomal enzymes in a reaction mixture contain many forms of reductase and oxygenase[11,12,16,17]. It is presumed that TFU might transform into 5-FU by hydrolyzing in the presence of NADPH-dependent reductase[18]. The activity of reductase is initiated by donating electrons from NADPH consumption[11,12]. The bio-transformation slowly releases 5-FU into the blood to keep the relatively long steady-state concentrations. 5-FU is then converted to deoxynucleotide (5-FdUMP) mediated by phosphoribosyl transferase in tumor cells and competes with deoxyuridine monophosphate (dUMP) for thymidylate synthetase[19-23]. DNA synthesis decreases, thus leading to cell death. Liver microsomal enzyme assay is currently underway to analyze the metabolism of sustained release of 5-FU.
In conclusion, TFU inhibits the growth of gastric carcinoma cells by sustaining the release of 5-FU. Liver microsomal enzymes mediate this bio-transformation. Experiments on a broad-spectrum of cancer cell lines are in progress.
S- Editor Liu Y L- Editor Wang XL E- Editor Liu WF
| 1. | Martinez J, Martin C, Chacon M, Korbenfeld E, Bella S, Senna S, Richardet E, Coppola F, Bas C, Hidalgo J. Irinotecan, oxaliplatin plus bolus 5-fluorouracil and low dose folinic acid every 2 weeks: a feasibility study in metastatic colorectal cancer patients. Am J Clin Oncol. 2006;29:45-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Sassi G, Striano B, Merlo UA. A reporting system for the assessment of chemotherapy toxicity. J Oncol Pharm Pract. 2005;11:63-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Wu SL, Sun ZJ, Yu L, Meng KW, Qin XL, Pan CE. Effect of resveratrol and in combination with 5-FU on murine liver cancer. World J Gastroenterol. 2004;10:3048-3052. [PubMed] |
| 4. | Schmidt Laugesen C, Steffansen B, Scherfig E, la Cour M. Pharmacokinetics of intravitreal 5-fluorouracil prodrugs in silicone oil: experimental studies in pigs. Acta Ophthalmol Scand. 2005;83:184-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Krishnaiah YS, Satyanarayana V, Dinesh Kumar B, Karthikeyan RS, Bhaskar P. In vivo pharmacokinetics in human volunteers: oral administered guar gum-based colon-targeted 5-fluorouracil tablets. Eur J Pharm Sci. 2003;19:355-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 73] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Berglund A, Carlsson G, Gustavsson B, Frödin JE, Ragnhammar P, Glimelius B. 5-FU split dose; a phase I/II and pharmacokinetic study of a different schedule of the Nordic regimen in advanced colorectal carcinoma. Anticancer Res. 2003;23:1789-1794. [PubMed] |
| 7. | El-Khoueiry AB, Lenz HJ. Should continuous infusion 5-fluorouracil become the standard of care in the USA as it is in Europe. Cancer Invest. 2006;24:50-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Xu W, Zhang Z, Castaner J. Atofluding. Drugs Fut. 2001;26:935-938. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 9. | Kametani T, Kigasawa K, Hiiragi M, Wakisaka K, Haga S, Nagamatsu Y, Sugi H, Fukawa K, Irino O, Yamamoto T. Studies on the synthesis of chemotherapeutics. 10. Synthesis and antitumor activity of N-acyl- and N-(alkoxycarbonyl)-5-fluorouracil derivatives. J Med Chem. 1980;23:1324-1329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Qu XJ, Yang JL, Russell PJ, Goldstein D. Changes in epidermal growth factor receptor expression in human bladder cancer cell lines following interferon-alpha treatment. J Urol. 2004;172:733-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Rastogi S, Das M, Khanna SK. A novel approach to study the activity and stoichiometry simultaneously for microsomal pentoxyresorufin-O-dealkylase reaction. FEBS Lett. 2002;512:121-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Das M, Rastogi S, Khanna SK. Mechanism to study 1: 1 stoichiometry of NADPH and alkoxyphenoxazones metabolism spectrophotometrically in subcellular biological preparations. Biochim Biophys Acta. 2004;1675:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Yang JL, Qu XJ, Russell PJ, Goldstein D. Regulation of epidermal growth factor receptor in human colon cancer cell lines by interferon alpha. Gut. 2004;53:123-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Cui SX, Qu XJ, Xie YY, Zhou L, Nakata M, Makuuchi M, Tang W. Curcumin inhibits telomerase activity in human cancer cell lines. Int J Mol Med. 2006;18:227-231. [PubMed] |
| 15. | Li Q, Feng FY, Han J, Sui GJ, Zhu YG, Zhang Y, Zhang ZH, Li L, Wang PH, Zhou MZ. [Phase III clinical study of a new anticancer drug atofluding]. Aizheng. 2002;21:1350-1353. [PubMed] |
| 16. | Park JY, Kim KA. Inhibitory effect of 5-fluorouracil on human cytochrome P(450) isoforms in human liver microsomes. Eur J Clin Pharmacol. 2003;59:407-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Peet CF, Enos T, Nave R, Zech K, Hall M. Identification of enzymes involved in phase I metabolism of ciclesonide by human liver microsomes. Eur J Drug Metab Pharmacokinet. 2005;30:275-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Simeonova M, Velichkova R, Ivanova G, Enchev V, Abrahams I. Study on the role of 5-fluorouracil in the polymerization of butylcyanoacrylate during the formation of nanoparticles. J Drug Target. 2004;12:49-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Pratt S, Shepard RL, Kandasamy RA, Johnston PA, Perry W 3rd, Dantzig AH. The multidrug resistance protein 5 (ABCC5) confers resistance to 5-fluorouracil and transports its monophosphorylated metabolites. Mol Cancer Ther. 2005;4:855-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 178] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 20. | Mizutani Y, Wada H, Yoshida O, Fukushima M, Nonomura M, Nakao M, Miki T. Significance of thymidylate synthase activity in renal cell carcinoma. Clin Cancer Res. 2003;9:1453-1460. [PubMed] |
| 21. | Peters GJ, Backus HH, Freemantle S, van Triest B, Codacci-Pisanelli G, van der Wilt CL, Smid K, Lunec J, Calvert AH, Marsh S. Induction of thymidylate synthase as a 5-fluorouracil resistance mechanism. Biochim Biophys Acta. 2002;1587:194-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 282] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 22. | Ma T, Zhu ZG, Ji YB, Zhang Y, Yu YY, Liu BY, Yin HR, Lin YZ. Correlation of thymidylate synthase, thymidine phosphorylase and dihydropyrimidine dehydrogenase with sensitivity of gastrointestinal cancer cells to 5-fluorouracil and 5-fluoro-2'-deoxyuridine. World J Gastroenterol. 2004;10:172-176. [PubMed] |
| 23. | Yamamoto S, Kubota K. Level of 5-fluorodeoxyuridine 5'-monophosphate in cancerous tissue in patients with gastric cancer under preoperative administration of TS-1. A preliminary study. J Exp Clin Cancer Res. 2005;24:457-462. [PubMed] |