Published online Nov 7, 2006. doi: 10.3748/wjg.v12.i41.6711
Revised: August 12, 2006
Accepted: September 22, 2006
Published online: November 7, 2006
AIM: To unravel the differences between systematic inflammatory response syndrome (SIRS) of acute pancreatitis compared to the same syndrome in sepsis.
METHODS: Twenty-five patients were enrolled, 12 with sepsis and 13 acute pancreatitis. After diagnosis 20 mL blood was sampled. Half were assayed for isolation of monocytes and 10 mL was centrifuged for serum test of tumor necrosis factor alpha (TNFα) and interleukin-6 (IL-6). Half of monocytes were incubated in the presence of patients’ serum and supernatants were collected. The other half was treated for estimation of optical photometry under caspase-3 inhibition. TNFα and IL-6 were estimated by an enzyme immunoassay.
RESULTS: median ± SE of serum IL-6 in septic patients and acute pancreatitis patients was 192.30 ± 35.40 ng/L and 21.00 ± 16.05 ng/L, respectively (P < 0.01). Respective values of caspase-3 were 0.94 ± 0.17 pmol/min 104 cells and 0.34 ± 0.09 pmol/min 104 cells (P < 0.05). IL-6 of monocyte supernatants of patients with sepsis was significantly increased after addition of patients’ serum, while that of patients with acute pancreatitis did not show significant difference.
CONCLUSION: The data have shown that monocyte activity is different between acute pancreatitis and sepsis. This phenomenon might be explained as a different pathway to the pro-inflammatory cytokines release or could be a novel anti-inflammatory response in acute pancreatitis.
- Citation: Koussoulas V, Tzivras M, Karagianni V, Spyridaki E, Plachouras D, Giamarellou H, Giamarellos-Bourboulis EJ. Monocytes in systematic inflammatory response syndrome: Differences between sepsis and acute pancreatitis. World J Gastroenterol 2006; 12(41): 6711-6714
- URL: https://www.wjgnet.com/1007-9327/full/v12/i41/6711.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i41.6711
Although the knowledge about the underlying pathogenic mechanisms in acute pancreatitis and sepsis has been considerably enriched, comparative studies are lacking. There is evidence that pro-inflammatory cytokine cascade plays a crucial role in both acute pancreatitis and sepsis[1]. Acute pancreatitis is an inflammation often complicated with systematic inflammatory response syndrome (SIRS). During the evolution to sepsis microbial components trigger serum monocytes, which release pro-inflammatory cytokines; and the latter leads to SIRS[2].
Septic cascade is thought to be initiated from pro-inflammatory cytokines released from blood monocytes[3]. Acute pancreatitis is one major cause of systemic inflammation attributed to the secretion of pro-inflammatory mediators but the contribution of monocytes to the biosynthesis of these mediators is not yet defined at least with data of the current literature. The aim of the present study was to unravel the differences of the ex vivo activity of monocytes upon occurrence of SIRS between patients with acute pancreatitis and sepsis.
Twenty-five patients were enrolled in a prospective study from January 2003 to June 2003; 13 patients were diagnosed with acute pancreatitis and 12 with sepsis. All were hospitalized in the 4th Department of Internal Medicine of the Medical School of Athens during that period. The study was conducted in accordance to the Helsinki Declaration.
Exclusion criteria for the study were the presence of (1) neutropenia (< 500 neutrophils/μL), (2) HIV infection, (3) corticosteroids administration for more than one month, defined as more than 1 mg/kg daily of equivalent prednisone, and (4) protein C administration at least five days before admission.
SIRS was defined by the presence of at least two of the followings[4]: (1) pulse rate > 90/min, (2) breath rate > 20/min or PCO2 < 32 mmHg, and (3) leukocytosis [white blood cells (WBCs) > 12 000/μL] or leucopenia (WBCs < 4000/μL) or more than 10% bands.
Inclusion criteria were the following for acute pancreatitis[5]: (1) SIRS, (2) initiation of a typical epigastric pain radiating to the back over the last 12 h before admission; (3) serum amylase at least three times above normal values, (4) urine amylase at least three times above normal values, (5) findings of acute edematous pancreatitis on ultrasound or on computed tomography, and (6) the absence of any primary liver disease.
Inclusion criteria for sepsis were the following in accordance to ASCP/SCCM 1997 classification[4]: (1) clinically proven infection with fever as its first manifestation over the last 12 h before admission, and (2) SIRS. Primary infections for sepsis were acute pyelonephritis, lower respiratory tract infection (LRTI) and acute cholangitis.
The diagnosis of acute pyelonephritis was made on the basis of the following criteria[6,7]: (1) a case history compatible with acute pyelonephritis comprising at least two spikes of fever above 38°C and presence of lumbar tenderness on examination, (2) pyuria defined as presence of more than 10 polymorphs per high power field under a light microscope, and (3) positive urinary culture with the colony count exceeding 104 cfu/mL for a single Gram-negative bacterial species. Diagnosis of LRTI was established in any patient presenting with the following signs: (1) core temperature > 38°C, and (2) new or persistent consolidation on lung X-ray[8]. Diagnosis of acute cholangitis was based on the following criteria: (1) core temperature > 38°C, (2) acute pain of upper right quadrant of abdomen elicited on palpation, and (3) ultrasound findings compatible with cholangitis[9].
Upon admission, a total of 20 mL of blood were collected after puncture of one forearm vein. Ten milliliters of blood were collected in a sterile heparinized syringe and the remaining in a sterile tube. Blood was centrifuged and the supernatant was kept at -70°C until assayed.
For the isolation of blood monocytes[10], the collected heparinized venous blood was layered over Ficoll Hypaque (Biochrom, Berlin, Germany) and centrifuged. Isolated mononuclear cells were washed three times with PBS (pH 7.2) (Merck, Darmsadt, Germany) and incubated with RPMI 1640 enriched with 10% FBS and 2 mmol/L of glutamine in the presence of 100 U/mL of penicillin G and 0.1 g/L of streptomycin (Sigma Co, St. Louis, USA) in flasks of 25 cm3. After one hour of incubation at 37°C in 5% CO2, non-adherent cells were removed; adherent monocytes were thoroughly washed with Hanks’ solution (Biochrom). Monocytes were then harvested with the application of a 0.25% trypsin/0.02% EDTA solution (Biochrom) and counted in a Neubauer plate. Their purity was more than 95% as determined after staining with the anti-CD14 monoclonal antibody at the fluorocolor FITC (emission 520 nm, Immunotech, Marseille, France) and reading through the EPICS XL/MSL flow cytometer (Beckman Coulter Co, Miami, Florida).
Half of the isolated monocytes were treated with an ice-cold cell lysis buffer (50 mmol/L HEPES, 0.1% CHAPS, 5 mmol/L DTT, 0.1 mmol/L EDTA, pH 7.4). After centrifugation for 10 min at 10 000 × g, and 4°C, activity of caspase-3 was estimated in the cytosolic extract by an enzymatic chromogenic assay (BIOMOL Research Laboratories, Plymouth, PA). It was based on the rate of hydrolysis at 37°C of a substrate releasing p-nitroaniline over-time, as assessed by sequential photometry at 405 nm. The assay was also performed in the presence of a caspase-3 inhibitor. The activity of caspase-3 in cell lysates was expressed as pmol/min 104 cells.
The remaining half of monocytes were distributed in two wells of a 12-well plate; they were incubated with RPMI 1640 supplemented with 10% FBS and 2 mmol/L of glutamine for 18 h at 37°C in 5% CO2 in the absence/presence of 0.1 mL of serum of the patient. The total volume of the added growth medium was 2.4 mL where patients’ sera represented 4.1%. After incubation, cell supernatants were collected and kept refrigerated at -70°Cuntil assayed.
Concentrations of TNFα and IL-6 in sera and monocyte supernatants were estimated by an enzyme immunoabsorbent assay (Diaclone, Paris, France). Lowest limits of detection were 0.5 pg/mL for TNFα and 6.25 ng/L for IL-6. Their concentrations in supernatants were expressed as pg/104 monocytes. TNFα and IL-6 and activity of caspase-3 were selected as parameters since the former two represented the considerable burden of pro-inflammatory cytokines secreted from monocytes in an early course of events leading to inflammation and the latter an index of apoptosis.
Concentrations of TNFα, IL-6 in sera and monocyte supernatants and caspase-3 intracellular activity were expressed as their median ± 95% confidence intervals of the mean (CI) or standard error of the mean (SE). Comparison between groups was made by Mann-Whitney U test. Correlations between concentrations of TNFα and IL-6 in serum, intracellular activity of caspase-3 and concentrations of TNFα and IL-6 of monocyte supernatants were performed according to Spearman’s rank of order. According to the estimated intracellular monocytic activity of caspase-3, patients were divided into those with activity lower and higher than 100 pmol/min 104 cells. Comparisons between sepsis and acute pancreatitis were performed by Pearson’s chi-square test. Any P value less than 0.05 was considered as significant.
mean ± SD age of enrolled patients with acute pancreatitis was 59.6 ± 18.5 years and of patients with sepsis 61.5 ± 17.6 years. Predisposing factors of pancreatitis were gallstones in eleven patients and hypertriglyceridemia in two patients. Primary infection for sepsis was acute pyelonephritis in six patients, LRTI in four patients and acute cholangitis in two patients.
mean ± SD APACHE II scores in patients with acute pancreatitis and sepsis were 7.68 ± 1.22 and 8.23 ± 1.48 respectively (P = NS). mean ± SD of Ranson Index score in patients with pancreatitis on admission and during the first 48 h after admission was 1.34 ± 0.32 and 1.69 ± 0.82 respectively.
median ± SE TNFα in serum was 11.61 ± 7.57 pg/mL and 17.01 ± 8.64 pg/mL, in patients with sepsis and acute pancreatitis, respectively (P = NS). Respective values for IL-6 were 192.30 ± 35.40 pg/mL and 21.00 ± 16.05 pg/mL (P < 0.01) and for intracellular activity of caspase 3, 0.94 ± 0.17 pmol/min 104 cells and 0.34 ± 0.09 pmol/min 104 cells, respectively. Occurrence of high-level caspase-3 monocytic intracellular activity was greater in patients with sepsis than in patients with acute pancreatitis (P < 0.05).
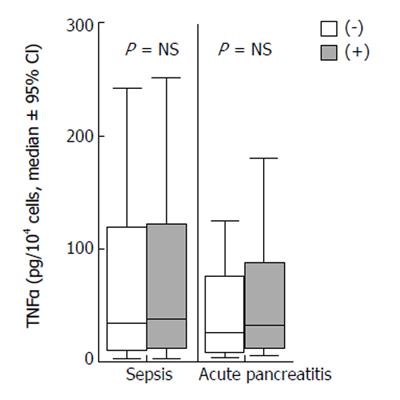
Concentrations of TNFα of monocyte supernatants of patients with sepsis and acute pancreatitis incubated in the absence or presence of patients’ sera are shown in Figure 1. No differences were found between yielded concentrations in the absence and presence of sera from either patients with sepsis or pancreatitis.
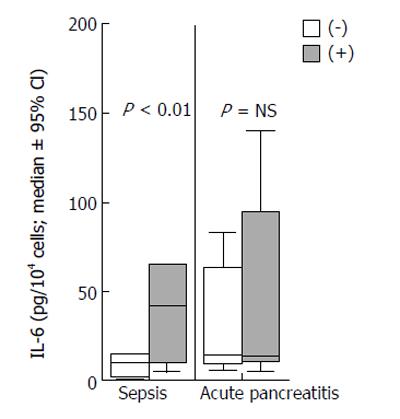
Concentrations of IL-6 of monocyte supernatants of patients with sepsis and acute pancreatitis incubated in the absence or presence of patients’sera are shown in Figure 2. The ratios of concentration of IL-6 in the presence of patients’ sera to concentration in the absence of patients’ sera were estimated. The ratio was significantly higher in sepsis than in acute pancreatitis (P < 0.01).
No correlation was found between intracellular activity of caspase-3 and TNFα and IL-6 of serum from either patients with acute pancreatitis or sepsis. Neither was correlation found between the intracellular activity of caspase-3 and either TNFα or IL-6 concentrations of monocyte supernatants in patients with either acute pancreatitis or sepsis.
Acute pancreatitis and sepsis represent two significant causes of mortality; subsequent multiple organ failure is thought to be the most severe complication of both[2]. SIRS is considered to result from pro-inflammatory cytokines released by the inflamed tissues or cells of the innate immune system. Recent data evidence for probable alterations of the function of cells of the innate immunity in both acute pancreatitis and sepsis. These changes are expressed as decreased expression of HLA-DR on cell membranes of monocytes[11,12]. Based on the latter evidence, the present study was focused on the comparative ex vivo activity of monocytes between patients with sepsis and acute pancreatitis.
It has been observed that most of the patients with acute pancreatitis develop organ dysfunction 48-72 h after initiation of their first clinical symptoms[13]. To our knowledge, the present study is the first attempting to investigate the probable role of monocytes in acute pancreatitis compared to their respective role in sepsis at an early time course; blood was sampled within 12 h after the first clinical symptom in both study groups[14].
Our results revealed that serum concentrations of IL-6 in patients with sepsis were significantly increased compared with that in patients with acute pancreatitis; similar finding was not observed for TNFα. The lack of similarities between TNFα and IL-6 might be attributed to the earlier release of TNFα in the pro-inflammatory cascade than that of IL-6[15].
TNFα of monocyte supernatants did not differ between sepsis and acute pancreatitis; it remained unaffected by the addition of patients’ serum (Figure 1). The lack of difference of TNFα release by monocytes between sepsis and pancreatitis might also be explained by its early production in the inflammatory cascade.
On the other hand, IL-6 secretion by monocytes was significantly increased in the presence of serum of patients with sepsis compared to that of patients with acute pancreatitis (Figure 2). Presence of serum in monocyte supernatants represented only 4% of the total culture volume; that percentage might be considered minimal to produce any confounding. A limitation of the study is the lack of evidence to explain the effect of serum and only hypotheses may be made. It might be presumed that an anti-inflammatory mediator was present in serum in patients with acute pancreatitis inhibiting further release of IL-6 from monocytes. That response might be implicated in sepsis also, but probably was inadequate to inhibit secretion of IL-6. However, it is of great significance that monocytes preserve secretion activity in acute pancreatitis; a finding seen for the first time.
Caspase-3 is involved in the intracellular apoptotic process[16]. Its intracellular activity was significantly increased in patients with sepsis compared to those with acute pancreatitis. Increased apoptosis of monocytes in sepsis may constitute an attempt of the host to reduce inflammatory responses. In patients with acute pancreatitis, apoptosis of monocytes was minimal. However, it might designate a late activation of monocytes in the evolution of acute pancreatitis compared to sepsis, as described by others[17].
The presented data reveal a different ex vivo activity of monocytes between sepsis and acute pancreatitis. This phenomenon might be explained by the existence of either of different pathways for the release of pro-inflammatory cytokines or of a novel anti-inflammatory activity of serum of patients with acute pancreatitis.
S- Editor Wang J L- Editor Zhu LH E- Editor Bi L
| 1. | Dib M, Zhao X, Wang X, Andersson E, Drewsen G, Andersson R. Acute phase response in acute pancreatitis: a comparison with abdominal sepsis. Scand J Gastroenterol. 2003;38:1072-1077. [RCA] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Pezzilli R, Ceciliato R, Barakat B, Corinaldesi R. Immune-manipulation of the inflammatory response in acute pancreatitis. What can be expected? JOP. 2004;5:115-121. [PubMed] |
| 3. | Raraty MG, Connor S, Criddle DN, Sutton R, Neoptolemos JP. Acute pancreatitis and organ failure: pathophysiology, natural history, and management strategies. Curr Gastroenterol Rep. 2004;6:99-103. [RCA] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 88] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 4. | Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med. 2003;29:530-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1609] [Cited by in RCA: 1677] [Article Influence: 76.2] [Reference Citation Analysis (0)] |
| 5. | Uhl W, Buchler MW, Malfertheiner P, Beger HG, Adler G, Gaus W. A randomised, double blind, multicentre trial of octreotide in moderate to severe acute pancreatitis. Gut. 1999;45:97-104. [RCA] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 119] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 6. | Giamarellos-Bourboulis EJ, Perdios J, Gargalianos P, Kosmidis J, Giamarellou H. Antimicrobial-induced endotoxaemia in patients with sepsis in the field of acute pyelonephritis. J Postgrad Med. 2003;49:118-122. [PubMed] |
| 7. | Ramakrishnan K, Scheid DC. Diagnosis and management of acute pyelonephritis in adults. Am Fam Physician. 2005;71:933-942. [PubMed] |
| 8. | Douzinas E, Giamarellos-Bourboulis EJ, Andrianakis I. Effect of intravenous administration of high doses of immunoglobulins on systemic endotoxaemia of patients with multiple injuries. Infect Dis Clin Pract. 2005;13:247-249. |
| 9. | Giamarellos-Bourboulis EJ, Skiathitis S, Dionyssiou-Asteriou A, Hatziantoniou S, Demetzos K, Dontas I, Papaioannou GT, Karatzas G, Helen G. Lipid peroxidation by Pseudomonas aeruginosa in the pathogenesis of nosocomial sepsis. J Postgrad Med. 2003;49:11-16; discussion 16. |
| 10. | Giamarellos-Bourboulis EJ, Plachouras D, Tzivra A, Kousoulas V, Bolanos N, Raftogiannis M, Galani I, Dontas I, Dionyssiou-Asteriou A, Giamarellou H. Stimulation of innate immunity by susceptible and multidrug-resistant Pseudomonas aeruginosa: an in vitro and in vivo study. Clin Exp Immunol. 2004;135:240-246. [RCA] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Richter A, Nebe T, Wendl K, Schuster K, Klaebisch G, Quintel M, Lorenz D, Post S, Trede M. HLA-DR expression in acute pancreatitis. Eur J Surg. 1999;165:947-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Pachot A, Monneret G, Brion A, Venet F, Bohe J, Bienvenu J, Mougin B, Lepape A. Messenger RNA expression of major histocompatibility complex class II genes in whole blood from septic shock patients. Crit Care Med. 2005;33:31-38; discussion 236-237. |
| 13. | Takeyama Y. Significance of apoptotic cell death in systemic complications with severe acute pancreatitis. J Gastroenterol. 2005;40:1-10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Curley P, Nestor M, Collins K, Saporoschetz I, Mendez M, Mannick JA, Rodrick ML. Decreased interleukin-2 production in murine acute pancreatitis: potential for immunomodulation. Gastroenterology. 1996;110:583-588. [RCA] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Imahara SD, O'Keefe GE. Genetic determinants of the inflammatory response. Curr Opin Crit Care. 2004;10:318-324. [RCA] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 46] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Chaturvedi V, Sitailo LA, Bodner B, Denning MF, Nickoloff BJ. Defining the caspase-containing apoptotic machinery contributing to cornification in human epidermal equivalents. Exp Dermatol. 2006;15:14-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Leindler L, Morschl E, Laszlo F, Mandi Y, Takacs T, Jarmai K, Farkas G. Importance of cytokines, nitric oxide, and apoptosis in the pathological process of necrotizing pancreatitis in rats. Pancreas. 2004;29:157-161. [RCA] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 2.6] [Reference Citation Analysis (0)] |