Published online Oct 28, 2006. doi: 10.3748/wjg.v12.i40.6546
Revised: July 12, 2006
Accepted: July 19, 2006
Published online: October 28, 2006
AIM: To determine the prevalence of celiac disease in a group of volunteer blood donors at a blood bank in the city of Curitiba, Brazil through detection of the serum marker immunoglobulin A (IgA) antitransglutaminase antibody.
METHODS: Blood samples collected from 2086 healthy subjects at the Paraná State Center for Hematology and Hemotherapy in Curitiba were submitted to ELISA testing for the IgA antitransglutaminase antibody. Positive samples received IgA antiendomysium antibody test through indirect immunofluorescence using human umbilical cord as substrate. Subsequently, patients who were positive on both tests underwent small bowel (distal duodenum) biopsy.
RESULTS: Six subjects, four males and two females, tested positive for the two serum markers. Five of the six were submitted to intestinal biopsy (one declined the procedure). Biopsy results revealed changes in the distal duodenum mucosa (three classified as Marsh IIIb lesions and two as Marsh II lesions). Most donors diagnosed having celiac disease presented multiple symptoms (gastrointestinal tract complaints). One donor reported having a family history of celiac disease (in a niece).
CONCLUSION: Among apparently healthy blood donors, the prevalence of biopsy-confirmed celiac disease was approximately 1:417, similar to that seen in European countries.
- Citation: Pereira MAG, Ortiz-Agostinho CL, Nishitokukado I, Sato MN, Damião AO, Alencar ML, Abrantes-Lemos CP, Cançado EL, Brito T, Ioshii SO, Valarini SB, Sipahi AM. Prevalence of celiac disease in an urban area of Brazil with predominantly European ancestry. World J Gastroenterol 2006; 12(40): 6546-6550
- URL: https://www.wjgnet.com/1007-9327/full/v12/i40/6546.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i40.6546
Celiac disease (CD) is a chronic disease that affects genetically susceptible individuals and is characterized by permanent intolerance to gluten and other related proteins, causing nonspecific, characteristic lesions in the small bowel mucosa[1]. These lesions prevent nutrient absorption in the affected area. Treatment involves a strict gluten-free diet (elimination of products containing wheat, barley, rye and probably oats). Clinical manifestations of CD are protean in nature and vary markedly with the age of the patient, the duration and extent of disease, and the presence of extraintestinal pathologic conditions. In addition to the classical gastrointestinal form, a variety of other clinical manifestations of the disease have been described, including atypical and asymptomatic forms[2].
Serologic tests developed over the past decades provide noninvasive tools for screening individuals at risk for the disease in both general and selected populations. However, for diagnosis of CD, it is necessary to perform histological evaluation in order to confirm intestinal damage in individuals presenting positive exam results.
Initially, the disease was considered a low-prevalence disorder and, consequently, diagnosis was based on clinical findings alone. Most prevalence studies of the disease have been carried out in European countries and have evaluated the prevalence based on symptomatic cases. Therefore, atypical and asymptomatic cases were frequently misdiagnosed. Currently, prevalence is higher due to the availability of reliable, easily administered serologic tests of high sensitivity and specificity.
In CD patients, a wide variety of clinical manifestations are seen, and the disease may be seen in conjunction with other diseases. Therefore, as well as being important tools for prevalence studies, serologic tests (performed prior to the mandatory intestinal biopsy used for diagnosis confirmation) have become important markers for the disease[3].
Studies carried out in Europe, USA and Brazil in order to investigate the prevalence of CD have shown that the prevalence of the disease is considerably higher than previously presumed. Recent studies of serum markers in blood donors have shown a prevalence of 1 to 250 in Sweden[4], 1 to 524 in Denmark[5], 1 to 333 in Holland[6], 1 to 157 in Israel[7] and 1 to 250 in the USA[8]. The first Brazilian epidemiologic study using serum markers was published in 2000 and showed the prevalence of CD to be 1 to 681 among blood donors in the national capital of Brasília[9].
In order to get further insight into the prevalence of CD in Brazil and to evaluate the influence of genetic and environmental predisposition, we chose the city of Curitiba (in the state of Paraná). The city is located in the southern part of Brazil, and its population is of mostly European ancestry. Eating habits in Curitiba are similar to those seen in the countries of origin, and the diet features a significant wheat component. We decided to evaluate the epidemiology of CD in blood donors at a blood bank and to simultaneously analyze the ethnic profile of these donors. To that end, we used serum markers and conducted a genealogical study.
We analyzed 2086 serum samples collected from healthy donors at the Paraná State Center for Hematology and Hemotherapy in Curitiba from January to December 2001, regardless of gender. Donor ages ranged from 20 to 62 (mean, 33 years). The Ethics Committee of the University of São Paulo School of Medicine Hospital das Clínicas and the Ethics Committee of the Paraná State Center for Hematology and Hemotherapy approved the study. Donors gave written informed consent and answered a standardized questionnaire on demographics and health. No significant disease was diagnosed, and all serologic tests for human immunodeficiency virus (HIV), hepatitis B, hepatitis C, and alanine transaminase were normal. Serum samples were centrifuged and stored at -80°C after serologic tests.
Serologic tests were performed in three University of São Paulo School of Medicine Medical Investigation Laboratories (LIM), LIM 06, LIM 07 and LIM 56. Samples positive for IgA antitissue transglutaminase (anti-tTG) by enzyme-linked immunosorbent assay (ELISA) were submitted to an immunofluorescence test for IgA antiendomysium antibody (EMA) using human umbilical cord as substrate. Each patient positive for both IgA anti-tTG and IgA EMA underwent small bowel (distal duodenum) endoscopic biopsy in which four samples were collected. This is the classical procedure accepted by the European Society of Pediatric Gastroenterology and Nutrition (ESPGAN)[10] for confirmation of a diagnosis of CD.
Technique carried out in accordance with the method described by Dieterich et al[11]: Microplates (96 wells; Corning, New York, NY, USA) were coated with 1 μg of guinea pig liver tTG (T 5398; Sigma, St. Louis, MO, USA) per well (activity: 1.5-3 KU/g of protein) in 100 µL Tris-HCl (50 mmol/L); NaCl (150 mmol/L) and CaCl2 (5 mmol/L), pH 7.5, and incubated at 37°C for 2 h. The wells were extensively rinsed in an Immunowash microplate washer (Bio-Rad, Hercules, CA, USA) in Tris-HCl (50 mmol/L), NaCl (150 mmol/L), EDTA (10 mmol/L) and Tween 20 (1 g/L), pH 7.4, and the microplates were incubated in washing buffer (100 μL/well) at 4°C overnight. The microplate solution was aspirated; serum samples were diluted (1/100) in washing buffer, poured into the respective wells (100 μL/well) and incubated for one hour at room temperature. After rinsing the plates (3 times), peroxidase-conjugated IgA-α antibody (A 0295, Sigma), diluted (1/1000) in washing buffer (100 μL/well), was added to the wells, which were then incubated at room temperature for one hour. Extensive rinsing eliminated unbound antibodies. The tetramethylbenzidine substrate (100 μL/well) promoted the color reaction. Microplates were placed in the dark for approximately 10 min, and subsequently blocked with 0.5 mol/L H2SO4 (50 μL/well). Absorbance values were read at 450 nm using an ELISA reader (VERSAmax tunable microplate reader; Molecular Devices, Sunnyvale, CA, USA). After subtraction of background values < 0.100, absorbance (A) readings were multiplied by the serum dilution in order to calculate the ELISA titers. With the inclusion of two control serum samples in every test, intra-assay variations (n = 22) and inter-assay variations (n = 24) were 8.68% and 8.38%, respectively. Statistical evaluation was carried out through receiver operator characteristic (ROC) curve analysis[12] using SPSS® software.
Immunofluorescence tests for antiendomysium antibodies were carried out using 2 μm cryosections of human umbilical cord, which were incubated with patient serum prediluted (initial dilution = 1:5) in buffer (PBS and 1 g/L Tween 80, pH 7.2), in a humid chamber at 37°C for 30 min. Slides were rinsed twice in PBS, pH 7.2, for 5 min. Samples were then incubated with fluorescein-conjugated anti-human IgA (Sigma) and diluted in dilution buffer (1:30). Subsequently, samples were rinsed twice with PBS and the slides were again incubated in humid chamber at 37°C for 30 min. Later, samples were read under fluorescence microscopy. Samples were considered positive if there was a hexagonal pattern of fluorescence throughout the peritubular muscle layer of the human umbilical cord vessels, marking the extracellular connective tissue.
Samples were fixed with buffered formalin and stained with hematoxylin and eosin (H&E) for histological study. The following aspects were evaluated: (1) crypt/villus ratio; (2) crypt regeneration; (3) characteristics of the inflammatory infiltrate in the section itself; (4) type of atrophy. Two pathologists examined every slide for the standardization of the histological aspects, using the histological classification developed in 1992 by Marsh and modified in 1997 by Rostami et al[13-15]. This modified system establishes five lesion classes. In Marsh 0, there is normal architecture of the mucosa and less than 40 intraepithelial lymphocytes per 100 enterocytes in the villus epithelium. Marsh I is defined as normal architecture of the mucosa and more than 40 lymphocytes per 100 enterocytes in the villus epithelium. Marsh II involves crypt enlargement (hyperplasia), in which immature epithelial cells are produced in large numbers and there is an influx of lymphocytes and plasmocytes. Under this system Marsh III has been reclassified and divided into three separate classes. In Marsh IIIa, there is partial villus atrophy combined with slight lymphocyte infiltration in epithelial cells and crypt hyperplasia. Marsh IIIb is marked by near total atrophy of the villi (villi still recognizable), crypt hyperplasia in which immature epithelial cells are produced in greater proportions, and influx of inflammatory cells. The final designation, Marsh IIIc, indicates total villus atrophy, hyperplasic crypts and infiltrative lesions[13-15].
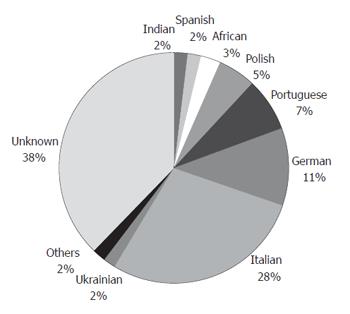
Of the 2086 blood donors, 1437 (68.88%) were males and 649 (31.12%) were females. Mean age was 33. There were 1977 Whites (94.77%), 82 Blacks (3.93%), and 27 Asians (1.30%). There were 1179 who claimed European ancestry (56.52%). Ethnic data were obtained through a genealogical study of the preceding three generations (Figure 1).
We identified six donors (four males and two females) who were positive for both anti-tTG and EMA. Five of these were submitted to intestinal biopsy and one declined the procedure. The procedure revealed that, in the mucosa of the small bowel (distal duodenum), three of the subjects presented Marsh IIIb lesions and two presented Marsh II lesions. Most subjects diagnosed with CD reported various gastrointestinal symptoms. One subject reported a family history of CD in a first-degree relative (a niece) (Table 1).
| Age | Gender | Race | Ancestry | GI symptoms | Anti-tTG1 | EMA1 | Histology |
| 26 | F | W | Italian and Portuguese | Diarrhea, distension, abdominal pain and flatulence | 1025 | 1/1280 | Marsh IIIb |
| 26 | F | W | Italian, Portuguese and German | Pyrosis, obstipation, epigastric pain, flatulence, weakness and fatigue | 295 | 1/640 | Marsh IIIb |
| 42 | M | W | Portuguese, German and Spanish | Pyrosis, obstipation and flatulence | 40 | 1/80 | Marsh II |
| 46 | M | W | Italian | Pyrosis | 2816 | 1/2560 | Marsh II |
| 57 | M | W | Italian and Portuguese | Diarrhea | 1540 | 1/640 | Marsh IIIb |
| 57 | M | W | Italian | Asymptomatic | 1840 | 1/640 | No biopsy |
The prevalence of biopsy-confirmed CD was approximately 1:417 among apparently healthy blood donors. When the cases were positive for antitransglu-taminase antibody were confirmed through the use of another marker, antiendomysium antibody, the prevalence was 1:347.
The sensitivity and specificity of the anti-tTG test were 100% and 96%, respectively. The OD cutoff value, established through analysis of the ROC curve, was 0.238. The area of the ROC curve was 0.999 ± 0.002.
In blood donors at a blood bank in Curitiba (Paraná), the prevalence of CD was 1:347 when samples positive for IgA anti-tTG antibodies were tested for a second marker (IgA antiendomysium antibodies). When subjects positive for both serum markers underwent distal duodenum biopsy, the prevalence was 1:417. This high prevalence is similar to that seen in European countries. This is unsurprising since most of the subjects in the study group were of European descent: 56.52% claimed European ancestry, and 37.78% were unaware of their ancestry (Figure 1). We can assume that most of the subjects of unknown ancestry were also of European descent since this ethnic group is predominant in Curitiba.
Factors that influence the high prevalence of CD are the genetic component (HLADQ2 and DQ8 haplotypes, which are strongly associated with CD and highly prevalent in the European population) and the consumption of grains containing gluten[2]. These two factors are present in the city of Curitiba, where, in recent decades, there has been an increase in the consumption of wheat flour. This increase is related to the lifting of sanctions on the importation of special wheat grains. In addition, the consumption of foods containing wheat has increased as a consequence of economic plans that have created conditions which facilitate consumption among the population[17]. Currently, wheat consumption in the south of Brazil, where Curitiba is located, is 61 kg/person per year-the highest in the country[17]. This corresponds to 43.57% of the consumption in Italy, which, according to the World Health Organization, is 140 kg/person per year.
Another relevant and notable aspect is that, of the six positive donors, five were of Italian descent, most from the region of Veneto (northern Italy). A study carried out in that region using serum marker screening showed a CD prevalence of 1:200[18].
The CD prevalence observed in the present study was higher than the 1:681 prevalence found in a similar study involving blood donors at a blood bank in the city of Brasília[9]. This low prevalence could be attributed to low numbers of individuals of European descent, although the actual ethnic composition of the Brasília population is not known. However, it is known that most of the population of the city migrated from the Central-West region of Brazil, where individuals of European ancestry are in the minority and the consumption of wheat is lower (23 kg/person per year)[17].
In the literature, the prevalence of CD is higher in females when studies are based on cases with clinical evidence[19]. However, recent studies of blood donors showed that, when the diagnosis is made through the use of serum markers, this relationship is not maintained[7,8]. In our study, approximately one-third of the participants were female, and we found the prevalence of CD to be similar between males and females and proportionate to their respective representation in the study population (four males and two females tested positive).
A prevalence study of CD in a population composed of the descendants of European immigrants to New Zealand was conducted in the city of Wellington[20]. The study was based on clinical findings in children and adults and showed that prevalences of the disease in children and adults were lower and the same as those seen in the respective European cities of origin. The authors attributed the lower prevalence in children to underdiagnosis. Although the population under study presented the same ancestry and possibly the same eating habits, the authors evaluated only “the tip of the celiac iceberg”. In the present study, which was conducted in a region of high wheat consumption and included a genealogical search and the use of serum markers, we evaluated “the rest of the iceberg”. Our results show that, when ethnic and environmental factors are constant, the prevalence of the disease remains stable, even if members of the population migrate to other areas. A recent study[21], conducted in Argentina, using serum markers to evaluate a population of European descent in an area with 85 kg/person per year of wheat consumption, showed a CD prevalence of 1:167, reinforcing our hypothesis.
In the present study, we found a prevalence of CD of 1:417 among apparently healthy blood donors. Our results demonstrate that the high prevalence of CD in the city of Curitiba is comparable to that seen in European countries. This finding supports the hypothesis that the prevalence of the disease remains stable if predisposing genetic and environmental conditions are maintained.
S- Editor Pan BR L- Editor Ma JY E- Editor Bi L
| 1. | Kagnoff MF. Overview and pathogenesis of celiac disease. Gastroenterology. 2005;128:S10-S18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 123] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 2. | Dewar DH, Ciclitira PJ. Clinical features and diagnosis of celiac disease. Gastroenterology. 2005;128:S19-S24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 135] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 3. | Romaldini CC, Barbieri D. [Serum antibodies in celiac disease]. Arq Gastroenterol. 1999;36:258-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 4. | Grodzinsky E, Franzen L, Hed J, Ström M. High prevalence of celiac disease in healthy adults revealed by antigliadin antibodies. Ann Allergy. 1992;69:66-70. [PubMed] |
| 5. | Weile B, Grodzinsky E, Skogh T, Jordal R, Cavell B, Krasilnikoff PA. Screening Danish blood donors for antigliadin and antiendomysium antibodies. Acta Paediatr Suppl. 1996;412:46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Rostami K, Mulder CJ, Werre JM, van Beukelen FR, Kerchhaert J, Crusius JB, Pena AS, Willekens FL, Meijer JW. High prevalence of celiac disease in apparently healthy blood donors suggests a high prevalence of undiagnosed celiac disease in the Dutch population. Scand J Gastroenterol. 1999;34:276-279. [RCA] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 64] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Shamir R, Lerner A, Shinar E, Lahat N, Sobel E, Bar-or R, Kerner H, Eliakim R. The use of a single serological marker underestimates the prevalence of celiac disease in Israel: a study of blood donors. Am J Gastroenterol. 2002;97:2589-2594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 84] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 8. | Not T, Horvath K, Hill ID, Partanen J, Hammed A, Magazzu G, Fasano A. Celiac disease risk in the USA: high prevalence of antiendomysium antibodies in healthy blood donors. Scand J Gastroenterol. 1998;33:494-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 240] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 9. | Gandolfi L, Pratesi R, Cordoba JC, Tauil PL, Gasparin M, Catassi C. Prevalence of celiac disease among blood donors in Brazil. Am J Gastroenterol. 2000;95:689-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 111] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 10. | Walker-Smith JA, Guandalini S, Schmitz J, Shmerling DH, Visakorpi JK. Revised criteria for diagnosis of coeliac disease. Report of Working Group of European Society of Paediatric Gastroenterology and Nutrition. Arch Dis Child. 1990;65:909-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1014] [Cited by in RCA: 1087] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 11. | Dieterich W, Laag E, Schopper H, Volta U, Ferguson A, Gillett H, Riecken EO, Schuppan D. Autoantibodies to tissue transglutaminase as predictors of celiac disease. Gastroenterology. 1998;115:1317-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 394] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 12. | Fletcher R, Fletcher SW, Wagner EH. Clinical epidemiology the essentials. 3th ed. Baltimore, Williams & Wilkins. 1984;53-102. |
| 13. | Rostami K, Kerckhaert J, Tiemessen R, von Blomberg BM, Meijer JW, Mulder CJ. Sensitivity of antiendomysium and antigliadin antibodies in untreated celiac disease: disappointing in clinical practice. Am J Gastroenterol. 1999;94:888-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 324] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 14. | Rostami K, von Blomberg BM, Meijer JWR. Antiendomysium antibodies indicate severity of villous atrophy. Eur J Gastroenterol Hepatol. 1997;9:A54. |
| 15. | Rostami K, Kerckhaert J, von Blomberg BM, Meijer JW, Wahab P, Mulder CJ. SAT and serology in adult coeliacs, seronegative coeliac disease seems a reality. Neth J Med. 1998;53:15-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Marsh MN. Gluten, major histocompatibility complex, and the small intestine. A molecular and immunobiologic approach to the spectrum of gluten sensitivity ('celiac sprue'). Gastroenterology. 1992;102:330-354. [PubMed] |
| 17. | Guth R; Personal communication. 2002;. |
| 18. | Volta U, Bellentani S, Bianchi FB, Brandi G, De Franceschi L, Miglioli L, Granito A, Balli F, Tiribelli C. High prevalence of celiac disease in Italian general population. Dig Dis Sci. 2001;46:1500-1505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 99] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 19. | Marsh MN. Coeliac disease. Oxford: Blackwell 1992; 49-80. |
| 20. | Ussher R, Yeong ML, Stace N. Coeliac disease: incidence and prevalence in Wellington 1985-92. N Z Med J. 1994;107:195-197. [PubMed] |
| 21. | Gomez JC, Selvaggio GS, Viola M, Pizarro B, la Motta G, de Barrio S, Castelletto R, Echeverría R, Sugai E, Vazquez H. Prevalence of celiac disease in Argentina: screening of an adult population in the La Plata area. Am J Gastroenterol. 2001;96:2700-2704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 115] [Article Influence: 4.8] [Reference Citation Analysis (0)] |