Published online Oct 28, 2006. doi: 10.3748/wjg.v12.i40.6420
Revised: August 12, 2006
Accepted: August 31, 2006
Published online: October 28, 2006
Mannose-binding lectin (MBL) is a pattern-recognition molecule that binds to characteristic carbohydrate motifs present on the surface of many different pathogens. MBL binding stimulates the immune system via the lectin pathway of complement activation. In certain clinical situations, often characterized by pre-existing immune compromise, MBL deficiency increases the risk of infectious and other disease-specific complications. Many of the key pathogenic processes inherent to common gastroenterological diseases, such as infection, immunological damage, and carcinogenesis, have been linked to MBL. This editorial reviews the biology of MBL, outlines key disease associations to document the breadth of influence of MBL, and finally, highlights the relevance of MBL to both gastroenterological health and disease.
- Citation: Worthley DL, Bardy PG, Gordon DL, Mullighan CG. Mannose-binding lectin and maladies of the bowel and liver. World J Gastroenterol 2006; 12(40): 6420-6428
- URL: https://www.wjgnet.com/1007-9327/full/v12/i40/6420.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i40.6420
Mannose-binding lectin (MBL) is an important component of the innate immune system. MBL is primarily produced by the liver, circulates throughout the body, and is able to recognize a wide array of common pathogens through repeating carbohydrate sequences present on microbial surfaces. MBL binding of pathogens initiates complement activation via the lectin pathway. There have been a large number of studies addressing the influence of MBL deficiency on infection, autoimmunity, and carcinogenesis, all critical processes in the pathogenesis of gastrointestinal disease. Genetically determined MBL deficiency increases the risk and manifestations of a wide range of diseases, particularly when the immune system is already compromised. This editorial provides an introduction to the structure, function and regulation of MBL and explores its clinical relevance, placing it in the context of common medical and, in particular, gastrointestinal conditions.
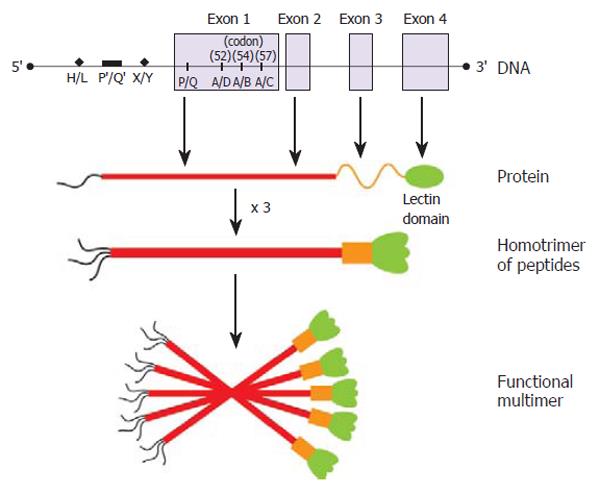
The capacity of MBL to recognize and eradicate pathogens is extremely variable. Within any given population there are individuals that have varying functional levels of circulating MBL. The relative sufficiency of MBL function for any given individual is largely determined by polymorphisms within the MBL2 gene, on chromosome 10. Three missense mutations within the first exon of MBL2 significantly effect MBL function (codon 54 ‘B’, codon 57 ‘C’, and codon 52 ‘D’) (Figure 1). These coding mutations are collectively designated ‘O’, and the wild-type, sufficient allele is represented by ‘A’. An Australian study of healthy blood donors found that the prevalence of MBL2 wild-type coding genotype A/A was 57.6%, coding mutation heterozygosity (A/O) was 34.8% (A/D 11%, A/B 19.9%, A/C 3.8%), and coding mutation homozygosity (O/O) was 7.6% (B/B 2.1%, B/C 2.1%, B/D 2.5%, D/D 0.9%)[1]. These frequencies are consistent with other Caucasoid populations[2-4]. In Asian communities, the most common mutation is also the ‘B’ allele, but the ‘D’ allele is virtually absent[5].
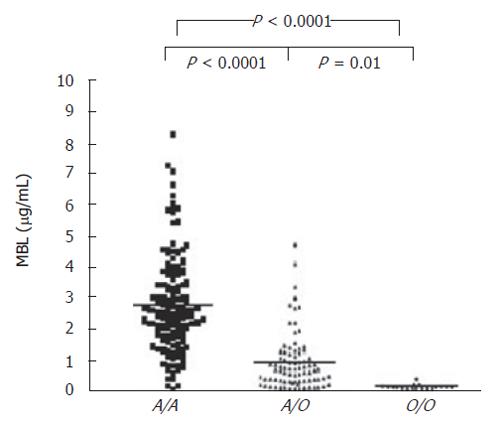
Further variability of MBL function is due, at least in part, to other polymorphisms within the promoter (position -550, G to C substitution, alleles ‘H’ and ‘L’ and position -221, G to C substitution, alleles ‘X’ and ‘Y’); and 5’-untranslated (position +4, C to T, alleles ‘P’ and ‘Q’) regions of the gene (Figure 1). When inherited in the context of a normal coding allele (A), the promoter region haplotypes HY, LY, and LX are associated with high, intermediate, and low serum MBL concentrations, respectively. The genotypes O/O, A/O and LXA/LXA, are all associated with low antigenic and functional levels of MBL (compared to A/A). The O/O genotype is correlated with the most extreme MBL deficit (Figure 2). Low levels of MBL associated with the common polymorphic variants appear to result from impaired oligomerization of the MBL triple helix (see below) into functional higher order multimers[6], as well as increased susceptibility to degradation by metalloproteinases[7].
The basic structural subunit of MBL is a homotrimer of MBL peptides, entwined in a triple helix (Figure 1). Each peptide contains a lectin domain to bind the specific oligosaccharide motifs present on the surface of many different microorganisms[8]. Functional MBL circulates as a higher-order multimer (tetramers, pentamers and hexamers) of the basic MBL subunit. This oligomerization allows high-affinity interaction between MBL and the microorganism. Binding of MBL to pathogens causes a conformational change in the MBL multimer, and activation of associated molecules, the MBL-associated serine proteases (MASPs), that initiate the lectin-complement pathway.
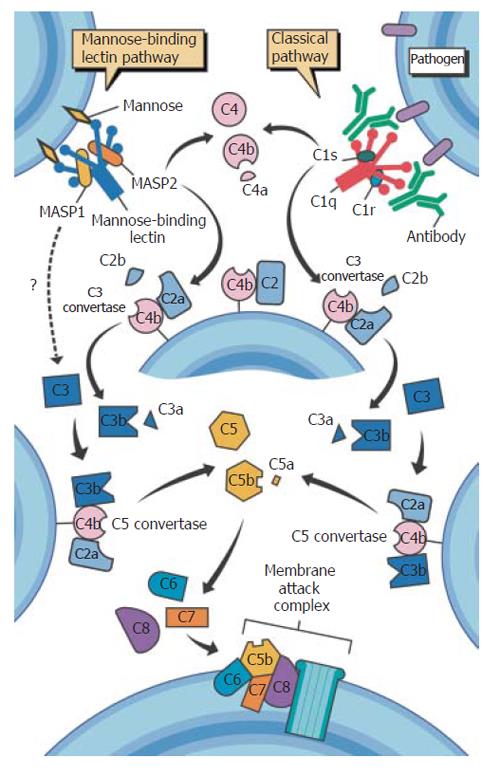
The enzymatic cascade of complement activation is a vital aspect of innate immunity. Complement-derived opsonization also provides an effective means of articulation with adaptive immunity through subsequent phagocytosis and antigen processing. The classical complement pathway is initiated by the binding of the C1 complex (C1q, r and s) to bound antibody on pathogen surfaces and the alternative pathway by binding of C3b to hydroxyl or amino groups on cell-surface molecules, as a result of spontaneous C3 turnover[9]. The lectin-complement pathway is the third arm of complement activation. Higher order MBL multimers circulate in a functional complex with three serine proteases MASP-1, MASP-2, MASP-3 and one non-protease molecule, MAp19[8]. This complex is analogous to the C1 complex that initiates the classical complement pathway, except that MBL binds to pathogens independently of antibody. Once activated, MASP-2, like its classical-pathway counterpart C1s, cleaves C4 to C4b, producing C4b2a, the C3 convertase. Subsequent production of C3b, also a key opsonin, generates the C5 convertase, which in turn produces the chemoattractant C5a, and, through C5b, the formation of the membrane-attack complex, C5b-C9 (Figure 3).
Recently, an additional mechanism of lectin-mediated complement activation, which bypasses the classical pathway proteins, has been described. Selander et al[10] demonstrated that an MBL-dependent alternative pathway mediated C3 deposition in C2 deficient serum. This bypass pathway may be of particular significance in the presence of complement deficiencies[11].
MBL binds a broad range of bacteria, viruses, fungi and protozoa (Table 1). Its affinity for Gram-negative and Gram-positive bacteria is mediated through cell surface components, such as lipopolysaccharide (endotoxin) and lipoteichoic acid, respectively. MBL deficiency increases in vivo susceptibility to many common bacterial infections, including Neisseria meningitidis[12], Streptococcus pneumoniae[13], and Staphylococcus aureus[14]. MBL deficiency may also increase the risk of several viral infections and some of the most compelling data in this area have been conducted in viral hepatitides, discussed below.
| Bacteria | Viruses | Fungi | Protozoa |
| Staphylococcus aureus | HIV–1 and 2 | Aspergillus fumigatus | Plasmodium falciparum |
| Streptococcus pneumoniae | Herpes simplex 2 | Candida albicans | Cryptospori-dium parvum |
| Streptococcus pyogenes | Influenza A | Cryptococcus neoformans | Trypanosoma cruzi |
| Enterococcus spp. | Hepatitis B virus | Saccharomyces cerevisiae | |
| Listeria monocytogenes | Hepatits C virus | ||
| Haemophilus influenzae | |||
| Neisseria meningitidis | |||
| Neisseria gonorrhoeae | |||
| Escherichia coli | |||
| Klebsiella spp. | |||
| Pseudomonas aeruginosa | |||
| Salmonella montevideo | |||
| Salmonella typhimurium | |||
| H pylori | |||
| Chlamydia trachomatis | |||
| Chlamydia pneumonia | |||
| Proprionibacterium acnes | |||
| Mycobacterium avium | |||
| Mycobacterium tuberculosis | |||
| Mycobacterium leprae | |||
| Leishmania chagasi | |||
The balance of evidence suggests that MBL deficiency is most relevant when immunity is already compromised as a consequence of immunological immaturity, for example in young children[15], or is impaired by comorbidity or medical therapy, such as in cystic fibrosis[16], after chemotherapy[17,18], or following transplantation[19,20]. In the pediatric population, MBL exerts greatest influence during an immunological “window of vulnerability”, between the decline in maternal passive immunity but before the development of a fully mature adaptive immune system. There is a strong association between MBL deficiency and childhood infection, which has been found for both milder respiratory tract infections managed within the community[21], as well as more severe infections requiring hospitalization[15]. In cystic fibrosis (CF), innate immunity is compromised in part by impaired mucociliary clearance and bronchiectasis. In one series of CF patients, those with mutant MBL2 alleles had worse pulmonary function and shorter survival to end-stage CF[16]. The same investigators reported successful MBL replacement in the management of one patient with rapidly progressive CF[22]. Several studies have shown an association between MBL deficiency and risk or severity of infection following chemotherapy[17,18].
A number of autoimmune disorders are associated with MBL. This may in part relate to the role of MBL in removing pathogens and apoptotic bodies, thus minimizing the emergence of cross-reactivity or auto-immunogenic epitopes[23]. Inherited deficiencies within the classical complement pathway predispose to systemic lupus erythematosus (SLE), thus it was logical to evaluate the role of MBL in this condition. A recent meta-analysis concluded that deficient MBL2 genotypes increase the risk of developing SLE[24]. Other studies have shown that MBL deficiency increases the risk of SLE-related complications, such as arterial thrombosis[25]. The effect of variant MBL and risk of vascular complications extend beyond patients with SLE. Several studies have now demonstrated an association between MBL2 mutations and risk of coronary artery disease[26-28]. These results have been supported by a population-based study from Denmark, that genotyped 9 245 individuals for MBL2 coding mutations[29]. Although MBL deficiency did not greatly increase the rate of morbidity or mortality within the population, those with biallelic mutations had a significantly greater risk of hospitalization for cardiovascular disease compared to those without deficient alleles [RR = 1.2 (1.0-1.4), P = 0.02][29].
To this point, all of the disease associations presented have identified the wild-type (A/A) MBL2 gene as advantageous. The global preservation of MBL2 -deficient haplotypes, however, hints at a selective advantage, at least under certain circumstances, of the deficient state. The concept of heterosis, whereby a heterozygous trait may demonstrate a selective advantage, has many well known examples, such as the ΔF508 mutation in the cystic fibrosis transmembrane conductance regulator (CFTR) gene and resistance to cholera toxin[30]. MBL-facilitated opsonization and phagocytosis could theoretically enhance the infectivity of some intracellular pathogens. The dichotomous nature of MBL deficiency is supported by several clinical studies that show it to be protective against several obligate intracellular organisms, including Mycobacterium leprae[31], M. tuberculosis[32], and Leishmania chagasi[33].
Both plasma-derived as well as recombinant MBL are now available for therapeutic use, albeit that the indications for replacement are still evolving. The results from the first phase I trial conducted in healthy, MBL-deficient individuals, has been published[34]. This study confirmed that MBL replacement is a technically viable option. Phase II trials are eagerly awaited.
Innate immunity has developed multiple strategies for protecting us against microbiological threats. Pattern-recognition molecules, such as toll-like receptors (TLR), NOD2/CARD15, and MBL, are particularly important in the alimentary tract, characterized by its large surface area and intimate relationship to the bowel contents, particularly the extreme microbial burden found within the colonic lumen. In addition to initiating inflammation, the capacity for immune tolerance is critical for normal bowel function. Although it is clear that the liver is the chief contributor to plasma MBL, mucosal MBL production may be relevant in localized immune defence, particularly within the alimentary tract[35]. The following examples present some of the better developed areas of gastroenterological MBL research, including inflammatory bowel disease, carcinogenesis, gastrointestinal and hepatotropic infection, and chronic liver disease.
Inflammatory bowel disease (IBD) is a pathological spectrum encompassing ulcerative colitis (UC), Crohn’s disease (CD), and indeterminate colitis. The resultant IBD phenotype is the consequence of multiple interactions between environmental factors, particularly enteric flora, and the host response to this environment, determined by immunogenetic, epithelial, and other non-immune genetic factors[36]. MBL, as an important component of innate immunity, has engendered considerable research interest. In an early study of 340 unrelated patients with IBD genotyped for MBL2 exon 1 coding mutations, the frequency of deficient alleles was significantly lower in patients with UC than either the control group (P = 0.02), or those with CD (P = 0.01)[37]. This study suggests that MBL deficiency could be protective against UC; alternatively, it could be interpreted that MBL deficiency, in individuals otherwise predisposed to IBD, may skew the phenotype away from the UC spectrum of disease towards CD. This concept is supported by another study that genotyped MBL2 in patients with CD, UC, or healthy controls[38]. In this study the allele frequency of coding mutations was approximately 30% in patients with CD, 8% in UC, and 16% in healthy controls. In addition, the frequency of homozygosity or compound heterozygosity for coding mutations (i.e. O/O MBL2 genotype) within the IBD group was significantly higher than in the healthy control population and the association was strengthened if the small number of UC patients were excluded from the analysis (16% vs 0%; P = 0.05)[38]. The study also assessed anti-Saccharomyces cerevisiae antibody (ASCA) and MBL levels within the same subsets of patients, albeit slightly different numbers within each group[38]. CD patients with MBL deficiency were significantly more likely to be positive for ASCA and for their lymphocytes to proliferate in response to mannan. Thus, it appears that MBL deficiency could impair normal processing of mannan-expressing microbial antigens, such as those found on the cell surface of many common microorganisms. The accumulated antigens could then stimulate the immune system, and contribute to the production of ASCA and possibly the pathogenesis of Crohn’s
disease[38]. ASCA is a well established phenotypic marker of IBD, tending to aggregate with Crohn’s rather than the ulcerative colitis phenotype, and within CD the presence of ASCA is particularly associated with the fibrostenosing phenotype and ileal inflammation[39]. Thus, MBL deficiency might act primarily to influence IBD-specific phenotype in these patients. It should be noted, however, that a follow-up study, testing a larger cohort of CD patients (n = 241), failed to confirm the significant association between variant MBL genotypes and ASCA positivity[40]. The observed trend, however, did show that the frequency of ASCA positivity was proportional to the relative deficiency of the coding genotype, with 54% ASCA positivity for A/A, 58% for A/O, and 67% for O/O[40]. Nevertheless, further studies into the role of MBL as a marker or regulator of IBD phenotype are warranted. Finally, the contribution of M. avium subspecies paratuberculosis to the pathogenesis of Crohn’s disease is controversial[41]. Nevertheless, it would be interesting to investigate whether, in a fashion analogous to M. tuberculosis, MBL might predispose to Crohn’s disease by facilitating the infectivity of this obligate intracellular pathogen.
Coeliac disease is an important autoimmune disorder involving the alimentary tract[42]. The majority of patients with coeliac disease express the major histocompatability complex (MHC) molecule DQ2 and the remainder usually carry DQ8[42]. But HLA genes convey only about 40% of the genetic risk, and although 30%-40% of Caucasians carry DQ2 or DQ8, less than 3% of these will develop coeliac disease[42]. In one study, 117 patients with histologically and serologically confirmed coeliac disease were genotyped for MBL2 exon 1 mutations, and compared to a healthy blood donor population. There was a significant difference in the frequency of the O/O genotype between those with coeliac disease (13%) and the control group (5%) (P = 0.04)[43]. A follow-up study included a detailed assessment of the coeliac disease patients’ MHC[23]. HLA susceptibility alleles and MBL2 exon 1 coding mutations were genotyped in 147 healthy controls and 149 patients with coeliac disease, enriched with 29 coeliac disease patients known to be negative for DQ2 and DQ8, which is extremely rare[23]. As in their first study, patients with coeliac disease had a greater frequency of the O/O genotype than healthy controls, but in addition, the association between coeliac disease and MBL deficiency was even stronger in the small number of patients negative for DQ2 and DQ8. It is likely that in those rare cases of coeliac disease that are negative for DQ2 and DQ8, the non-HLA susceptibility genotypes would exert a greater effect. Their study also analyzed apoptosis within small intestinal biopsy specimens, and showed that MBL tended to aggregate to areas of apoptosis within the epithelium. MBL has been implicated in the normal clearance of apoptotic bodies[44,45]. The authors postulated that the association between MBL and coeliac disease, and indeed other autoimmune conditions, could relate to impaired apoptosis, whereby MBL deficiency impairs the normal removal and clearance of apoptotic cells, that may subsequently reveal previously hidden self-antigen, causing loss of self-tolerance, and spreading of autoimmunity[23]. The association between variant MBL2 alleles and coeliac disease has also been confirmed within the Finnish population[46].
Experimentally there is the suggestion that MBL (both wild-type and the mutant B allele) may possess anti-colorectal cancer tumour activity[47]. In vitro MBL binds specifically to oligosaccharide moieties on colorectal cancer cell line SW1116[47]. The investigators transplanted SW1116 cells subcutaneously in nude mice, resulting in palpable tumor masses at three weeks. In order to evaluate the in vivo anti-tumoral activity of MBL, the mice were administered one of four different intra-tumoral injections. The first group received an injection with vaccinia virus carrying the wild-type (‘A’) MBL2 allele, the second group with the variant ‘B’ allele and the two control groups received vaccinia virus alone, or saline alone. Intra-tumoral administration of the recombinant vaccinia virus carrying a MBL2 gene (either the ‘A’ or ‘B’ allele) significantly reduced tumor size as compared with the two control groups (P < 0.005), and also prolonged survival[47]. These laboratory results have not, however, been reflected in clinical trials. In fact, patients with colorectal cancer have increased activation of the lectin-complement pathway and increased levels of serum MBL[48]. In patients undergoing surgery for colorectal cancer, however, low preoperative levels of serum MBL has been linked to an increased risk of developing post-colectomy pneumonia[49]. Most recently, increased preoperative serum levels of MASP2 predicted adverse outcome following colorectal cancer surgery, both in terms of disease recurrence (P = 0.03; HR = 1.4, 1.0-2.0) and survival (P = 0.0005; HR = 1.4, 1.2-1.7)[50]. There are several possible explanations for these results. A preoperative elevation in acute phase markers, such as CRP, is known to predict worse outcome[51], and the elevation in MASP2 may simply reflect a heightened inflammatory state. Alternatively, MASP2 may meaningfully influence tumor progression. Further studies are required to clarify the role of the lectin-complement pathway in cancer.
Despite the well-established role of MBL in innate immunity, there have been relatively few studies detailing the clinical effect of MBL deficiency in enteric infections. One notable exception analyzed the association between MBL deficiency and risk of Cryptosporidium parvum enteritis. This study included 72 African patients with acquired immunodeficiency syndrome (AIDS) and diarrhea. They were genotyped for exon 1 MBL2 mutations and had their duodenal aspirates tested for MBL. Patients with biallelic coding mutations (O/O) had a significantly greater chance of cryptosporidiosis compared to those who were either wild-type or heterozygous for MBL2 mutation (A/A or A/O, respectively) (OR = 8.2; 95% CI: 1.5-42; P = 0.02)[52]. This study places MBL’s anti-microbial function back in the context of the ‘window of vulnerability’ hypothesis. Of further interest from this study was the detection of MBL within some of the duodenal aspirates. The presence of albumin in the intestinal lumen led the authors to postulate that MBL entered the bowel through mucosal leakage of serum; however, local intestinal production could not be excluded. The association between MBL deficiency and cryptosporidiosis was recently confirmed in a second case-control study, this time in young (< 3 year) Haitian children. Mean serum MBL levels were significantly lower in the cases (1110 vs 2395 ng/mL, P = 0.002), and 37% of the cases compared to only 10% of the healthy controls were found to be deficient in MBL (level ≤ 70 ng/mL) (P = 0.005)[53]. Unlike the earlier study, MBL2 genotyping was not performed, and thus MBL deficiency secondary to enteric protein loss, as a consequence of cryptosporidiosis, could not be excluded. When considered together, however, these two studies present compelling evidence for the role of MBL in the host defense against Cryptosporidium spp. infection.
Another study analyzed serum MBL levels in a pediatric population presenting with Escherichia coli 0157: H7 colitis. MBL levels were measured in patients with uncomplicated E. coli 0157:H7 colitis, patients in whom the colitis was complicated by haemolytic uraemic syndrome (HUS), and in normal and disease (rotavirus enteritis) control groups[54]. MBL deficiency was not associated with an increased risk of either infection nor the complication of HUS, albeit that without analysis of MBL2 genotype, overall MBL status may be more difficult to assess.
H pylori is one of the most common human bacterial infections, affecting approximately 50% of humans, although only 10%-20% of those affected will develop a clinical disorder[55]. Several immunogenetic polymorphisms are associated with clinical outcomes in H pylori infection[56], as well as with the risk of infection itself[57,58].
H pylori activates MBL in vitro[59], and a recent study demonstrated that H pylori-related chronic gastritis causes an increase in gastric mucosal MBL expression, but no association was found between MBL2 genotype and risk of chronic gastritis[60]. A recent study was performed to investigate whether MBL deficiency increased the risk of H pylori infection[61]. Two normal populations (166 blood donors and 108 stem cell donors) were included in the analysis. All individuals were genotyped for MBL2, had their peripheral MBL activity characterized by level and functional assays, and were tested for serological evidence of H pylori infection. In this study, MBL deficiency did not increase the risk of H pylori infection, and in one population greater MBL activity actually increased the risk of infection.
It is worth noting that MBL has been implicated in mediating gastrointestinal ischemia/reperfusion injury in mice[62]. MBL-null mice (deficient in the two murine genes encoding MBL) developed only minor gut injury after induced ischemia/reperfusion insult compared to the wild-type mice. MBL has been implicated as a mediator of ischemia/reperfusion injury in both the myocardium[63] and the kidney[64] and thus clinical correlation of MBL status and risk or outcome following mesenteric ischemia may yield interesting results.
MBL was first isolated from hepatocytes[65], and the liver produces most if not all of the circulating MBL[20]. There is obviously considerable functional reserve in hepatic MBL production, because in the setting of cirrhosis, unlike many other hepatic proteins, MBL production appears to be increased[66]. The viral hepatitides have stimulated considerable MBL-related research. In one study of chronic hepatitis B virus (HBV) infection, MBL codon 54 (B) mutations were significantly associated with risk of developing both symptomatic cirrhosis and spontaneous bacterial peritonitis (SBP)[67]. The increased risk of SBP in patients with MBL deficiency is biologically plausible, given that low levels of ascitic fluid opsonins are important in the pathogenesis of SBP, and MBL deficiency would be likely to compound this deficit[68]. A second study by the same investigators recently confirmed the association, extending the results from their previous study, to include the low expression haplotype XA as a risk factor, as well as the ‘B’ allele. The odds ratio for developing cirrhosis and hepatocellular carcinoma was 1.97 for patients with XA and 1.90 for those with YB (P = 0.002)[69]. A study from the U.S. confirmed these findings, which is important given that the age and route of acquisition of HBV may vary between different countries[70]. It is likely that MBL plays an important role in the pathogenesis of HBV-related chronic disease, even though some small studies have failed to confirm the association[71,72]. It will be interesting to examine the influence of MBL status upon the rate or type of drug resistance that emerges in individuals during long-term antiviral therapy.
Many of the studies analyzing MBL in chronic hepatitis C virus (HCV) infection have investigated the role of MBL mutations on rate of sustained viral response following interferon alpha (IFN-α) monotherapy. Two studies, from the same Japanese group, reported that patients who failed to eradicate HCV following IFN monotherapy were more likely to have variant MBL2 alleles, either the ‘B’ coding mutation[73,74] or the ‘LXPA’ haplotype[73]. A third Japanese study addressed whether MBL deficiency altered the course of HCV chronic liver disease[75]. In their cross-sectional study, 52 patients with chronic HCV and 50 controls were genotyped for the ‘B’ coding mutation in exon 1 of MBL2. All patients with HCV had the stage and activity of their liver disease categorized as “chronic inactive hepatitis”, “chronic active hepatitis” (CAH), or cirrhosis. No significant differences in the frequency of mutations was found between the patients and the controls, but within the HCV-infected group, all of the patients with heterozygous or homozygous codon 54 mutations had either CAH or cirrhosis, whilst none of those in the “chronic inactive hepatitis” group had mutations. This represented a significantly higher frequency of mutation in the advanced (CAH plus cirrhosis) liver disease group (P = 0.0405)[75]. A final study from Scotland sought to test the Japanese findings in a Caucasoid population[76]. This study failed to find an association between MBL deficiency and either progression of liver disease or response to IFN-α therapy. This study, however, did not perform MBL2 genotyping, but stratified MBL concentrations into four groupings for comparison. On balance, there appears to be an association between MBL status and HCV in terms of both disease progression and response to monotherapy, at least in the Japanese population. The development of newer anti-viral treatment regimens, including pegylated-IFN in combination with ribavirin treatment, makes it necessary to re-evaluate immunogenetic influences, at least, any which are hoped to inform therapy.
One of the more exciting recent reports regarding MBL and hepatobiliary disease, addressed the role of MBL deficiency following orthotopic liver transplantation (OLT)[20]. OLT provides the unique opportunity not only to evaluate the role of MBL in post-transplant infection, but also to assess the contribution of hepatic and extra-hepatic MBL production, because in many recipients the MBL2 genotype will be different in the liver. The study reported the clinical results from 49 transplants, in which 49 of the donors were genotyped for MBL2, 25 of the recipients were genotyped, and serum samples were collected from 25 of the recipients to evaluate the change in serum MBL concentration post-transplant. There was an impressive correlation between the risk of post-transplant clinically significant infection and the relative deficiency of the donor MBL2 genotype, with infection occurring in 12% receiving a wild-type A/A liver, 39% of those receiving an A/O liver, and in 67% of those receiving an O/O donor liver (P = 0.01)[20]. In addition, the post-transplantation serum MBL level was predicted by the hepatic, not the extra-hepatic, genotype. Nevertheless, as only 25 of the recipients, and thus only 25 extra-hepatic genotypes were analyzed, this study was too small to detect more subtle changes in the risk of infection, conferred through local, extra-hepatic MBL production[77].
The site of MBL production has been a contentious area. Undoubtedly, the liver produces the majority of MBL and most if not all of the circulating MBL within peripheral blood. This view is supported by the liver transplantation study above, as well as a report documenting that successful allo-SCT failed to correct peripheral blood MBL deficiency[20,78]. Nevertheless, MBL mRNA is expressed in extra-hepatic tissue[35,79,80] possibly including haemopoietic lineages[35], and there have now been two allo-SCT studies that support a contribution from donor (i.e. hematopoietic) MBL2 genotype, and risk of post-transplant infection[4,81]. The most important issue is not whether extra-hepatic MBL production significantly influences peripheral blood MBL levels, but whether it contributes in a clinically meaningful way to local, tissue-specific immunity.
MBL has stimulated a great deal of basic and clinical gastroenterological research and has provided new insights to the pathogenesis of infectious and immune disorders within the bowel and liver. The possibility of a local, mucosal effect of MBL as suggested by gene expression[35,79,80], as well as clinical studies[23,60] is an exciting discovery. Further work is needed to clarify whether mucosal production occurs, and if so whether it contributes to local immune surveillance in health, or under certain situations, even exacerbates alimentary tract disease.
Despite the promise of replacement therapy and the value of MBL in predicting the risk of disease and disease-specific complications, for now investigation of MBL status remains primarily a research tool. Future studies more rigorously examining MBL status by both measurement of MBL levels and MBL2 genotyping in large patient cohorts will help clarify the most important disease associations and identify those clinical settings in which MBL replacement therapy is most likely to be beneficial. Now that MBL replacement has been shown to be feasible, the first trials of MBL replacement therapy in several clinical settings, including recurrent infection, severe sepsis, and liver transplantation, are likely to be reported in the next few years. This prospect of MBL replacement therapy represents the culmination of several decades of basic and translational research and is an exciting advance in the field of innate immunity.
S- Editor Liu Y L- Editor Kumar M E- Editor Ma WH
| 1. | Minchinton RM, Dean MM, Clark TR, Heatley S, Mullighan CG. Analysis of the relationship between mannose-binding lectin (MBL) genotype, MBL levels and function in an Australian blood donor population. Scand J Immunol. 2002;56:630-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 150] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 2. | Madsen HO, Garred P, Thiel S, Kurtzhals JA, Lamm LU, Ryder LP, Svejgaard A. Interplay between promoter and structural gene variants control basal serum level of mannan-binding protein. J Immunol. 1995;155:3013-3020. [PubMed] |
| 3. | Mullighan CG, Heatley S, Bardy PG, Lester S, Rischmueller M, Gordon TP. Lack of association between mannose-binding lectin gene polymorphisms and primary Sjogren’s syndrome. Arthritis Rheum. 2000;43:2851-2852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Mullighan CG, Heatley S, Doherty K, Szabo F, Grigg A, Hughes TP, Schwarer AP, Szer J, Tait BD, Bik To L. Mannose-binding lectin gene polymorphisms are associated with major infection following allogeneic hemopoietic stem cell transplantation. Blood. 2002;99:3524-3529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 149] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 5. | Ip WK, To YF, Cheng SK, Lau YL. Serum mannose-binding lectin levels and mbl2 gene polymorphisms in different age and gender groups of southern Chinese adults. Scand J Immunol. 2004;59:310-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Larsen F, Madsen HO, Sim RB, Koch C, Garred P. Disease-associated mutations in human mannose-binding lectin compromise oligomerization and activity of the final protein. J Biol Chem. 2004;279:21302-21311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 176] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 7. | Butler GS, Sim D, Tam E, Devine D, Overall CM. Mannose-binding lectin (MBL) mutants are susceptible to matrix metalloproteinase proteolysis: potential role in human MBL deficiency. J Biol Chem. 2002;277:17511-17519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Holmskov U, Thiel S, Jensenius JC. Collections and ficolins: humoral lectins of the innate immune defense. Annu Rev Immunol. 2003;21:547-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 604] [Cited by in RCA: 566] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 9. | Medzhitov R, Janeway C Jr. Innate immunity. N Engl J Med. 2000;343:338-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1517] [Cited by in RCA: 1450] [Article Influence: 58.0] [Reference Citation Analysis (0)] |
| 10. | Selander B, Mårtensson U, Weintraub A, Holmström E, Matsushita M, Thiel S, Jensenius JC, Truedsson L, Sjöholm AG. Mannan-binding lectin activates C3 and the alternative complement pathway without involvement of C2. J Clin Invest. 2006;116:1425-1434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 138] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 11. | Atkinson JP, Frank MM. Bypassing complement: evolutionary lessons and future implications. J Clin Invest. 2006;116:1215-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Bax WA, Cluysenaer OJ, Bartelink AK, Aerts PC, Ezekowitz RA, van Dijk H. Association of familial deficiency of mannose-binding lectin and meningococcal disease. Lancet. 1999;354:1094-1095. [RCA] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 39] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Roy S, Knox K, Segal S, Griffiths D, Moore CE, Welsh KI, Smarason A, Day NP, McPheat WL, Crook DW. MBL genotype and risk of invasive pneumococcal disease: a case-control study. Lancet. 2002;359:1569-1573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 210] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 14. | Shi L, Takahashi K, Dundee J, Shahroor-Karni S, Thiel S, Jensenius JC, Gad F, Hamblin MR, Sastry KN, Ezekowitz RA. Mannose-binding lectin-deficient mice are susceptible to infection with Staphylococcus aureus. J Exp Med. 2004;199:1379-1390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 226] [Cited by in RCA: 222] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 15. | Summerfield JA, Sumiya M, Levin M, Turner MW. Association of mutations in mannose binding protein gene with childhood infection in consecutive hospital series. BMJ. 1997;314:1229-1232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 263] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 16. | Garred P, Pressler T, Madsen HO, Frederiksen B, Svejgaard A, Høiby N, Schwartz M, Koch C. Association of mannose-binding lectin gene heterogeneity with severity of lung disease and survival in cystic fibrosis. J Clin Invest. 1999;104:431-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 305] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 17. | Neth O, Hann I, Turner MW, Klein NJ. Deficiency of mannose-binding lectin and burden of infection in children with malignancy: a prospective study. Lancet. 2001;358:614-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 210] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 18. | Peterslund NA, Koch C, Jensenius JC, Thiel S. Association between deficiency of mannose-binding lectin and severe infections after chemotherapy. Lancet. 2001;358:637-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 190] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 19. | Mullighan CG, Bardy PG. Mannose-binding lectin and infection following allogeneic hemopoietic stem cell transplantation. Leuk Lymphoma. 2004;45:247-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Bouwman LH, Roos A, Terpstra OT, de Knijff P, van Hoek B, Verspaget HW, Berger SP, Daha MR, Frölich M, van der Slik AR. Mannose binding lectin gene polymorphisms confer a major risk for severe infections after liver transplantation. Gastroenterology. 2005;129:408-414. [PubMed] |
| 21. | Koch A, Melbye M, Sorensen P, Homoe P, Madsen HO, Molbak K, Hansen CH, Andersen LH, Hahn GW, Garred P. Acute respiratory tract infections and mannose-binding lectin insufficiency during early childhood. JAMA. 2001;285:1316-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 310] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 22. | Garred P, Pressler T, Lanng S, Madsen HO, Moser C, Laursen I, Balstrup F, Koch C, Koch C. Mannose-binding lectin (MBL) therapy in an MBL-deficient patient with severe cystic fibrosis lung disease. Pediatr Pulmonol. 2002;33:201-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 77] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 23. | Boniotto M, Braida L, Baldas V, Not T, Ventura A, Vatta S, Radillo O, Tedesco F, Percopo S, Montico M. Evidence of a correlation between mannose binding lectin and celiac disease: a model for other autoimmune diseases. J Mol Med (Berl). 2005;83:308-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Lee YH, Witte T, Momot T, Schmidt RE, Kaufman KM, Harley JB, Sestak AL. The mannose-binding lectin gene polymorphisms and systemic lupus erythematosus: two case-control studies and a meta-analysis. Arthritis Rheum. 2005;52:3966-3974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 137] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 25. | Øhlenschlaeger T, Garred P, Madsen HO, Jacobsen S. Mannose-binding lectin variant alleles and the risk of arterial thrombosis in systemic lupus erythematosus. N Engl J Med. 2004;351:260-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 156] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 26. | Madsen HO, Videm V, Svejgaard A, Svennevig JL, Garred P. Association of mannose-binding-lectin deficiency with severe atherosclerosis. Lancet. 1998;352:959-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 122] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 27. | Rugonfalvi-Kiss S, Endresz V, Madsen HO, Burian K, Duba J, Prohaszka Z, Karadi I, Romics L, Gonczol E, Fust G. Association of Chlamydia pneumoniae with coronary artery disease and its progression is dependent on the modifying effect of mannose-binding lectin. Circulation. 2002;106:1071-1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 64] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Best LG, Davidson M, North KE, MacCluer JW, Zhang Y, Lee ET, Howard BV, DeCroo S, Ferrell RE. Prospective analysis of mannose-binding lectin genotypes and coronary artery disease in American Indians: the Strong Heart Study. Circulation. 2004;109:471-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 80] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 29. | Dahl M, Tybjaerg-Hansen A, Schnohr P, Nordestgaard BG. A population-based study of morbidity and mortality in mannose-binding lectin deficiency. J Exp Med. 2004;199:1391-1399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 117] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 30. | Gabriel SE, Brigman KN, Koller BH, Boucher RC, Stutts MJ. Cystic fibrosis heterozygote resistance to cholera toxin in the cystic fibrosis mouse model. Science. 1994;266:107-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 308] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 31. | Garred P, Harboe M, Oettinger T, Koch C, Svejgaard A. Dual role of mannan-binding protein in infections: another case of heterosis. Eur J Immunogenet. 1994;21:125-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 145] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 32. | Søborg C, Madsen HO, Andersen AB, Lillebaek T, Kok-Jensen A, Garred P. Mannose-binding lectin polymorphisms in clinical tuberculosis. J Infect Dis. 2003;188:777-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 115] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 33. | Santos IK, Costa CH, Krieger H, Feitosa MF, Zurakowski D, Fardin B, Gomes RB, Weiner DL, Harn DA, Ezekowitz RA. Mannan-binding lectin enhances susceptibility to visceral leishmaniasis. Infect Immun. 2001;69:5212-5215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 108] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 34. | Valdimarsson H, Vikingsdottir T, Bang P, Saevarsdottir S, Gudjonsson JE, Oskarsson O, Christiansen M, Blou L, Laursen I, Koch C. Human plasma-derived mannose-binding lectin: a phase I safety and pharmacokinetic study. Scand J Immunol. 2004;59:97-102. [RCA] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 74] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 35. | Seyfarth J, Garred P, Madsen HO. Extra-hepatic transcription of the human mannose-binding lectin gene (mbl2) and the MBL-associated serine protease 1-3 genes. Mol Immunol. 2006;43:962-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 76] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 36. | Lakatos PL, Fischer S, Lakatos L, Gal I, Papp J. Current concept on the pathogenesis of inflammatory bowel disease-crosstalk between genetic and microbial factors: pathogenic bacteria and altered bacterial sensing or changes in mucosal integrity take "toll". World J Gastroenterol. 2006;12:1829-1841. [PubMed] |
| 37. | Rector A, Lemey P, Laffut W, Keyaerts E, Struyf F, Wollants E, Vermeire S, Rutgeerts P, Van Ranst M. Mannan-binding lectin (MBL) gene polymorphisms in ulcerative colitis and Crohn's disease. Genes Immun. 2001;2:323-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 38. | Seibold F, Konrad A, Flogerzi B, Seibold-Schmid B, Arni S, Juliger S, Kun JF. Genetic variants of the mannan-binding lectin are associated with immune reactivity to mannans in Crohn’s disease. Gastroenterology. 2004;127:1076-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 39. | Bossuyt X. Serologic markers in inflammatory bowel disease. Clin Chem. 2006;52:171-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 140] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 40. | Joossens S, Pierik M, Rector A, Vermeire S, Ranst MV, Rutgeerts P, Bossuyt X. Mannan binding lectin (MBL) gene polymorphisms are not associated with anti-Saccharomyces cerevisiae (ASCA) in patients with Crohn's disease. Gut. 2006;55:746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 41. | Naser SA, Ghobrial G, Romero C, Valentine JF. Culture of Mycobacterium avium subspecies paratuberculosis from the blood of patients with Crohn’s disease. Lancet. 2004;364:1039-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 429] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 42. | Green PH, Jabri B. Celiac disease. Annu Rev Med. 2006;57:207-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 122] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 43. | Boniotto M, Braida L, Spanò A, Pirulli D, Baldas V, Trevisiol C, Not T, Tommasini A, Amoroso A, Crovella S. Variant mannose-binding lectin alleles are associated with celiac disease. Immunogenetics. 2002;54:596-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 44. | Nauta AJ, Raaschou-Jensen N, Roos A, Daha MR, Madsen HO, Borrias-Essers MC, Ryder LP, Koch C, Garred P. Mannose-binding lectin engagement with late apoptotic and necrotic cells. Eur J Immunol. 2003;33:2853-2863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 247] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 45. | Ogden CA, deCathelineau A, Hoffmann PR, Bratton D, Ghebrehiwet B, Fadok VA, Henson PM. C1q and mannose binding lectin engagement of cell surface calreticulin and CD91 initiates macropinocytosis and uptake of apoptotic cells. J Exp Med. 2001;194:781-795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 895] [Cited by in RCA: 866] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 46. | Iltanen S, Mäki M, Collin P, Mustalahti K, Kaukinen K, Partanen J, Hulkkonen J, Hurme M, Aittoniemi J. The association between mannan-binding lectin gene alleles and celiac disease. Am J Gastroenterol. 2003;98:2808-2809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 47. | Ma Y, Uemura K, Oka S, Kozutsumi Y, Kawasaki N, Kawasaki T. Antitumor activity of mannan-binding protein in vivo as revealed by a virus expression system: mannan-binding proteindependent cell-mediated cytotoxicity. Proc Natl Acad Sci USA. 1999;96:371-375. [RCA] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 94] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 48. | Ytting H, Jensenius JC, Christensen IJ, Thiel S, Nielsen HJ. Increased activity of the mannan-binding lectin complement activation pathway in patients with colorectal cancer. Scand J Gastroenterol. 2004;39:674-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 66] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 49. | Ytting H, Christensen IJ, Jensenius JC, Thiel S, Nielsen HJ. Preoperative mannan-binding lectin pathway and prognosis in colorectal cancer. Cancer Immunol Immunother. 2005;54:265-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 50. | Ytting H, Christensen IJ, Thiel S, Jensenius JC, Nielsen HJ. Serum mannan-binding lectin-associated serine protease 2 levels in colorectal cancer: relation to recurrence and mortality. Clin Cancer Res. 2005;11:1441-1446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 51. | Nozoe T, Matsumata T, Kitamura M, Sugimachi K. Significance of preoperative elevation of serum C-reactive protein as an indicator for prognosis in colorectal cancer. Am J Surg. 1998;176:335-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 181] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 52. | Kelly P, Jack DL, Naeem A, Mandanda B, Pollok RC, Klein NJ, Turner MW, Farthing MJ. Mannose-binding lectin is a component of innate mucosal defense against Cryptosporidium parvum in AIDS. Gastroenterology. 2000;119:1236-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 64] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 53. | Kirkpatrick BD, Huston CD, Wagner D, Noel F, Rouzier P, Pape JW, Bois G, Larsson CJ, Alston WK, Tenney K. Serum mannose-binding lectin deficiency is associated with cryptosporidiosis in young Haitian children. Clin Infect Dis. 2006;43:289-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 54. | Proulx F, Wagner E, Toledano B, Decaluwe H, Seidman EG, Rivard GE. Mannan-binding lectin in children with Escherichia coli O157: H7 haemmorrhagic colitis and haemolytic uraemic syndrome. Clin Exp Immunol. 2003;133:360-363. [PubMed] |
| 55. | Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347:1175-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1848] [Cited by in RCA: 1907] [Article Influence: 82.9] [Reference Citation Analysis (3)] |
| 56. | El-Omar EM, Carrington M, Chow WH, McColl KE, Bream JH, Young HA, Herrera J, Lissowska J, Yuan CC, Rothman N. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000;404:398-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1690] [Cited by in RCA: 1676] [Article Influence: 67.0] [Reference Citation Analysis (0)] |
| 57. | Thye T, Burchard GD, Nilius M, Müller-Myhsok B, Horstmann RD. Genomewide linkage analysis identifies polymorphism in the human interferon-gamma receptor affecting Helicobacter pylori infection. Am J Hum Genet. 2003;72:448-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 76] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 58. | Zambon CF, Basso D, Navaglia F, Germano G, Gallo N, Milazzo M, Greco E, Fogar P, Mazza S, Di Mario F. Helicobacter pylori virulence genes and host IL-1RN and IL-1beta genes interplay in favouring the development of peptic ulcer and intestinal metaplasia. Cytokine. 2002;18:242-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 59. | Kuipers S, Aerts PC, van Dijk H. Differential microorganism-induced mannose-binding lectin activation. FEMS Immunol Med Microbiol. 2003;36:33-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 60. | Bak-Romaniszyn L, Cedzyński M, Szemraj J, St Swierzko A, Zeman K, Kałuzyński A, Płaneta-Małecka I. Mannan-binding lectin in children with chronic gastritis. Scand J Immunol. 2006;63:131-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 61. | Worthley DL, Smith A, Bampton PA, Cole SR, Young GP. Many participants in fecal occult blood test population screening have a higher-than-average risk for colorectal cancer. Eur J Gastroenterol Hepatol. 2006;18:1079-1083. |
| 62. | Hart ML, Ceonzo KA, Shaffer LA, Takahashi K, Rother RP, Reenstra WR, Buras JA, Stahl GL. Gastrointestinal ischemia-reperfusion injury is lectin complement pathway dependent without involving C1q. J Immunol. 2005;174:6373-6380. [PubMed] |
| 63. | Walsh MC, Bourcier T, Takahashi K, Shi L, Busche MN, Rother RP, Solomon SD, Ezekowitz RA, Stahl GL. Mannose-binding lectin is a regulator of inflammation that accompanies myocardial ischemia and reperfusion injury. J Immunol. 2005;175:541-546. [PubMed] |
| 64. | Møller-Kristensen M, Wang W, Ruseva M, Thiel S, Nielsen S, Takahashi K, Shi L, Ezekowitz A, Jensenius JC, Gadjeva M. Mannan-binding lectin recognizes structures on ischaemic reperfused mouse kidneys and is implicated in tissue injury. Scand J Immunol. 2005;61:426-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 123] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 65. | Summerfield JA, Taylor ME. Mannose-binding proteins in human serum: identification of mannose-specific immunoglobulins and a calcium-dependent lectin, of broader carbohydrate specificity, secreted by hepatocytes. Biochim Biophys Acta. 1986;883:197-206. [RCA] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 56] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 66. | Homann C, Garred P, Hasselqvist P, Graudal N, Thiel S, Thomsen AC. Mannan-binding protein and complement dependent opsonization in alcoholic cirrhosis. Liver. 1995;15:39-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 67. | Yuen MF, Lau CS, Lau YL, Wong WM, Cheng CC, Lai CL. Mannose binding lectin gene mutations are associated with progression of liver disease in chronic hepatitis B infection. Hepatology. 1999;29:1248-1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 68. | Bhuva M, Ganger D, Jensen D. Spontaneous bacterial peritonitis: an update on evaluation, management, and prevention. Am J Med. 1994;97:169-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 69. | Chong WP, To YF, Ip WK, Yuen MF, Poon TP, Wong WH, Lai CL, Lau YL. Mannose-binding lectin in chronic hepatitis B virus infection. Hepatology. 2005;42:1037-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 76] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 70. | Thio CL, Mosbruger T, Astemborski J, Greer S, Kirk GD, O'Brien SJ, Thomas DL. Mannose binding lectin genotypes influence recovery from hepatitis B virus infection. J Virol. 2005;79:9192-9196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 63] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 71. | Hohler T, Wunschel M, Gerken G, Schneider PM, Meyer zum Buschenfelde KH, Rittner C. No association between mannose-binding lectin alleles and susceptibility to chronic hepatitis B virus infection in German patients. Exp Clin Immunogenet. 1998;15:130-133. [RCA] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 72. | Bellamy R, Ruwende C, McAdam KP, Thursz M, Sumiya M, Summerfield J, Gilbert SC, Corrah T, Kwiatkowski D, Whittle HC. Mannose binding protein deficiency is not associated with malaria, hepatitis B carriage nor tuberculosis in Africans. QJM. 1998;91:13-18. [RCA] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 91] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 73. | Matsushita M, Hijikata M, Matsushita M, Ohta Y, Mishiro S. Association of mannose-binding lectin gene haplotype LXPA and LYPB with interferon-resistant hepatitis C virus infection in Japanese patients. J Hepatol. 1998;29:695-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 74. | Matsushita M, Hijikata M, Ohta Y, Iwata K, Matsumoto M, Nakao K, Kanai K, Yoshida N, Baba K, Mishiro S. Hepatitis C virus infection and mutations of mannose-binding lectin gene MBL. Arch Virol. 1998;143:645-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 61] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 75. | Sasaki K, Tsutsumi A, Wakamiya N, Ohtani K, Suzuki Y, Watanabe Y, Nakayama N, Koike T. Mannose-binding lectin polymorphisms in patients with hepatitis C virus infection. Scand J Gastroenterol. 2000;35:960-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 76. | Kilpatrick DC, Delahooke TE, Koch C, Turner ML, Hayes PC. Mannan-binding lectin and hepatitis C infection. Clin Exp Immunol. 2003;132:92-95. [PubMed] |
| 77. | Worthley DL, Bardy PG, Angus P, Harley H, Mullighan CG. Mannose-binding lectin and liver transplantation. Gastroenterology. 2005;129:1805-1806; author reply 1806-1807. [PubMed] |
| 78. | Kilpatrick DC, Stewart K, Allan EK, McLintock LA, Holyoake TL, Turner ML. Successful haemopoietic stem cell transplantation does not correct mannan-binding lectin deficiency. Bone Marrow Transplant. 2005;35:179-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 79. | Wagner S, Lynch NJ, Walter W, Schwaeble WJ, Loos M. Differential expression of the murine mannose-binding lectins A and C in lymphoid and nonlymphoid organs and tissues. J Immunol. 2003;170:1462-1465. [PubMed] |
| 80. | Uemura K, Saka M, Nakagawa T, Kawasaki N, Thiel S, Jensenius JC, Kawasaki T. L-MBP is expressed in epithelial cells of mouse small intestine. J Immunol. 2002;169:6945-6950. [PubMed] |
| 81. | Granell M, Urbano-Ispizua A, Lozano F, Rovira M, Fernandez-Aviles F, Ortega M, Uriburu C, Talarn C, Martínez C, Guardia A. Donor’s Mannan-Binding Lectin (MBL) Gene Polymorphism Is Associated with Invasive Fungal Infection Following Allogeneic Stem Cell Transplantation. Blood (ASH Annual Meeting Abstracts). 2004;104:2220. |
| 82. | Worthley DL, Bardy PG, Mullighan CG. Mannose-binding lectin: biology and clinical implications. Intern Med J. 2005;35:548-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 120] [Article Influence: 6.0] [Reference Citation Analysis (0)] |