Published online Jan 28, 2006. doi: 10.3748/wjg.v12.i4.636
Revised: July 8, 2005
Accepted: July 25, 2005
Published online: January 28, 2006
AIM: To evaluate the hepatoprotective effect of garlic on liver injury induced by isoniazid (INH) and rifampicin (RIF).
METHODS: Wistar rats weighing 150-200 g were treated orally with 50 mg/kg of INH and RIF daily each for 28 d. For hepatoprotective studies, 0.25 g/kg per day of freshly prepared garlic homogenate was administered orally half an hour before the INH+RIF doses. Serum alanine aminotransferase (ALT), aspartate aminotransferase (AST) and bilirubin were estimated on d 0, 14, 21, and 28 in all the rats. Histological analysis was carried out to assess the injury to the liver. Lipid peroxidation (LPO) as a marker of oxidative stress and non-protein thiols (glutathione) for antioxidant levels were measured in liver homogenate.
RESULTS: The treatment of rats with INH+RIF (50 mg/kg per day each) induced hepatotoxicity in all the treated animals as judged by elevated serum ALT, AST, and bilirubin levels, presence of focal hepatocytic necrosis (6/8) and portal triaditis (8/8). Garlic simultaneously administered at a dose of 0.25 g/kg per day prevented the induction of histopathological injuries in INH+RIF co-treated animals, except in 4 animals, which showed only moderate portal triaditis. The histological changes correlated with oxidative stress in INH+RIF treated animals. The group which received 0.25 g/kg per day garlic homogenate along with INH+RIF showed higher levels of glutathione (P < 0.05) and low levels of LPO (P < 0.05) as compared to INH+RIF treated group.
CONCLUSION: Freshly prepared garlic homogenate protects against INH+RIF-induced liver injury in experimental animal model.
- Citation: Pal R, Vaiphei K, Sikander A, Singh K, Rana SV. Effect of garlic on isoniazid and rifampicin-induced hepatic injury in rats. World J Gastroenterol 2006; 12(4): 636-639
- URL: https://www.wjgnet.com/1007-9327/full/v12/i4/636.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i4.636
Tuberculosis (TB) is one of the most common infectious diseases. In India, pulmonary tuberculosis is one of the major causes for adult deaths[1]. INH and RIF, the first line drugs used for tuberculosis chemotherapy, are associated with hepatotoxicity[2]. The rate of hepatotoxicity has been reported to be much higher in developing countries like India (8%-30%) compared to that in advanced countries (2%-3%) with a similar dose schedule[3]. We have established in our laboratory oxidative stress as one of the mechanisms for INH+RIF-induced hepatic injury[4]. Majority of normally formed free radicals are removed by the action of reduced glutathione. In circumstances where there is a reduction in glutathione results in the initiation of lipid peroxidation (LPO) resulting in tissue injury[5]. Garlic, an antioxidant, has been shown to inhibit LPO[6] and dose-dependent induction of endogenous antioxidants in rat kidney and liver[7]. Hence this protocol was designed to study the protective effect of garlic on INH+RIF-induced liver injury in Wistar rats.
Wistar rats weighing 150-200 g body were used in the study. The protocol was approved by the Institute’s Animal Ethical Committee. Body weights of these rats were monitored sequentially in control and experimental animals for a period of 28 d. Animals were divided into four groups as control (n = 8), INH+RIF (n = 8), garlic (n = 8), and INH+RIF+garlic (n = 12) where n was the number of animals included in the study.
For hepatotoxic model, 50 mg/kg per day of INH and RIF each was used in the study[8]. INH and RIF solutions were prepared separately in sterile distilled water. The pH of RIF solution was adjusted to 3.0 with 0.1 mol/L HCl[9]. INH and RIF were administered orally for 28 d. Liver transaminases and bilirubin were estimated on d 0, 14, 21, and 28 in both control (saline treated) and experimental animals. The criteria for hepatotoxicity were the presence of histological changes such as hepatocytic necrosis and portal triaditis along with elevated transaminases (more than thrice the upper normal limit). For the hepatoprotective model, 0.25 g/kg per day of freshly prepared garlic homogenate along with INH+RIF solution was administered[10].
For the preparation of fresh garlic homogenate, garlic bulbs were purchased from a local supermarket, cut into small pieces and homogenized in a motor-driven Teflon glass homogenizer on ice.
Rats were killed after the INH+RIF and garlic treatment. Representative blocks of liver were taken and fixed in 40g/L formoldehyde. Light microscopic examination of the liver was done on sections stained with (H) and (E). Special stains like reticulin and Ziehl Nielsen for acid fast bacilli were carried out whenever necessary. Various slides of different groups (two slides per animal) containing full cross-sections of the major hepatic lobules were prepared.
Twelve hours after the last oral treatment, rats were killed by cervical dislocation. Liver was excised immediately, quickly cooled and perfused with cold normal saline. Ten percent homogenate was prepared by homogenizing the liver tissue in 100 mmol/L potassium phosphate, 150 mmol/L potassium chloride buffer containing 200g/L glycerol (pH 7.5). LPO in tissue homogenate was measured by the reaction of LPO products like malondialdehyde (MDA) with thiobarbituric acid by the method of Kornbrust and Mavis[11].
Estimation of thiols (non-protein thiols) was carried out in tissue homogenate according to the method of Sedlak and Lindsay[12]. Protein estimation was done by the method of Lowry et al[13].
Data were analyzed by analysis of variance (ANOVA) followed by multiple comparisons using Dunnett’s procedure to compare all groups against control, Student-Newman-Keuls procedure was used to compare all the groups’ pair wise.
There was no mortality in any of the groups. The body weight and relative liver weights of the experimental animals calculated at the end of the study had no statistically significant difference when compared to the control animals (Table 1).
| Treatment (n) | Mortality(dead/total) | Body weight(g) | Relativeliver weight (g) |
| Control (8) | 0/8 | 153 ± 29 | 4.65 ± 0.32 |
| INH+RIF (8) | 0/8 | 150 ± 31 | 4.27 ± 0.41 |
| Garlic (8) | 0/8 | 148 ± 31 | 4.51 ± 0.40 |
| INH+RIF+garlic (12) | 0/12 | 159 ± 31 | 4.69 ± 0.29 |
Three-fold rise above the normal upper limits in the measured serum transaminases in INH+RIF group on d 28 of the experiment was a biochemical indication of liver injury. Garlic-treated and control animals had normal values of transaminase (Table 2). However, there was a significant increase in serum bilirubin in all the animals of INH+RIF group (Table 2) and in four animals of INH+RIF+garlic group in whom histological changes were present. Serum bilirubin was not significantly different in control, garlic and INH+RIF+garlic groups in whom histological changes were not present at the end of the study.
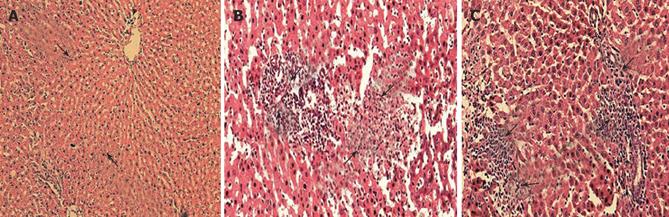
The treatment of rats with INH+RIF (50 mg/kg per day each) induced hepatotoxicity in all the treated animals as judged by elevated serum ALT, AST, and bilirubin levels, portal triaditis (8/8) and liver necrosis (Figure 1A and 1B). Simultaneously administered garlic at a dose of 0.25 g/kg per day prevented the induction of histopathological injuries in INH+RIF co-treated animals (8/12), except in four animals, who showed only moderate portal triaditis (Figure 1C).
The INH+RIF-administered animals exhibited significantly low levels of hepatic non-protein thiols (P < 0.01) as compared to control (Table 3) and co-administration of garlic and INH+RIF showed increased levels in hepatoprotected group as compared to control (P < 0.01). Fresh garlic homogenate by itself did not affect hepatic non-protein thiols significantly as compared to control. Treatment with INH+RIF depleted significantly the content of non-protein thiols as compared to control (P < 0.001, Table 3). Co-administration of freshly prepared garlic homogenate maintained its levels in the liver protected group although less than that in the control group (P < 0.05).
The treatment with INH+RIF significantly enhanced the peroxidation of lipids (P < 0.01) and co-administration of freshly prepared garlic homogenate blocked the induction of LPO caused by INH+RIF treatment. Freshly prepared garlic homogenate-treatment alone did not reduce the LPO levels (Table 3) in rats.
In the present study, hepatotoxicity model in Wistar rats was successfully produced by administering INH and RIF (50 mg/kg per day each) orally. The protective effect of 0.25 g/kg per day freshly prepared garlic homogenate on INH+RIF induced liver injury in rats was evaluated as previously described[8]. We did not study the metabolites of administered dose of garlic in the blood of INH+RIF-treated rats because absorption and metabolism of allicin-derived compounds are only partially understood. Allicin and allicin-derived compounds, including diallyl sulfides (DAS), ajoene and vinyldithins have never been detected in human blood, urine or stools even after the consumption of fresh garlic up to 25 g or 60 mg of pure allicin[14].
Garlic bulb contains approximately 65% water, 28% carbohydrates (mainly fructans), 2.3% organosulfur compounds, 2% protein (mainly alliinase), 1.2% free amino acids (mainly arginine) and 1.5% fiber. Intact garlic bulbs contain a high amount of γ-glutamylcysteine. These reserve compounds can be hydrolyzed and oxidized to form alliin, which accumulates naturally during storage of garlic bulbs at cool temperature. After various kinds of processing, such as cutting, crushing, chewing or dehydration, the vacuolar enzyme, alliinase, rapidly lyses cytosolic cysteine sulfoxides (alliin) to form the odoriferous alkyl alkane-thiosulfinates such as allicin. Allicin and other thiosulfinates instantly decompose to other compounds, such as DAS, diallyl disulfide (DADS), and diallyltrisulfide (DATS), dithiins and ajoene. At the same time, γ-glutamylcysteines are converted to S-allyl cysteine (SAC) via a pathway other than the alliin/allicin pathway[15].
Garlic has been found to have an important dietary and medicinal role for centuries. Most of its prophylactic and therapeutic effects are ascribed to specific oil and water soluble organosulfur compounds. Thiosulfinates and other secondary metabolites of garlic, including steroids, terpenoids, flavonoids and other phenols, may be responsible for reported therapeutic effects of garlic. Reuter et al[16] have reviewed the therapeutic effects of garlic on cardiovascular system as well as its antibiotic, anticancer, antioxidant, immunomodulatory, anti-inflammatory, hypoglycemic and hormone-like effects. Garlic also increases anti-inflammatory monocyte IL-10 production and decreases proinflammatory cytokines such as TNF-α, IL-1β, IL-6, IL-8, T cell interferon gamma, IL-2[17].
Garlic, a natural substance, has also been shown to inhibit LPO[18]. Phytochemicals from plant rich diets (including garlic) provide an important additional protection against oxidative damage[19]. There are a variety of antioxidants in garlic, which protect against disease-causing oxidative damage[20]. Garlic and related organosulfur compounds have antioxidant, detoxifying and other properties. These detoxifying effects are related to their ability to inhibit phase I enzymes and induce phase II enzymes or bind to exogenous toxins through sulfhydryl groups[21]. Previous studies on the mechanism of INH+RIF-induced hepatotoxicity have shown that non-protein thiols play a very important role in the detoxification of reactive toxic metabolites of INH+RIF. Liver injury has been observed when glutathione stores are markedly depleted[22]. The present study has further strengthened the protective role of garlic in INH+RIF-induced hepatic non-protein thiol depletion. These observations may be due to the inhibition of bioactivation of INH+RIF metabolites resulting in the decreased formation of INH electrophiles. A similar protective role of garlic has been documented in acetaminophen-induced hepatotoxicity[23].
Non-protein thiol is an important defense mechanism in living cells. As a substrate for antioxidant enzymes, i.e. glutathione peroxidase and glutathione reductase, it protects cellular constituents from the damaging effects of peroxidase formed in metabolism and other reactive oxygen species reactions. Aged garlic extract increases cellular glutathione in a variety of cells including those in normal liver and mammary tissue[24]. In the present study, the oxidative injury induced by INH and RIF could be prevented by fresh garlic homogenate. Thus this study represents a novel and an attractive idea to prevent INH+RIF-induced hepatic injury by co-administration of fresh garlic homogenate.
S- Editor Wang XL, Pan BR and Guo SY L- Editor Elsevier HK E- Editor Bi L
| 2. | Tasduq SA, Peerzada K, Koul S, Bhat R, Johri RK. Biochemical manifestations of anti-tuberculosis drugs induced hepatotoxicity and the effect of silymarin. Hepatol Res. 2005;31:132-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 70] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 3. | Sharma SK. Antituberculosis drugs and hepatotoxicity. Infect Genet Evol. 2004;4:167-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Sodhi CP, Rana SV, Mehta SK, Vaiphei K, Attari S, Mehta S. Study of oxidative-stress in isoniazid-rifampicin induced hepatic injury in young rats. Drug Chem Toxicol. 1997;20:255-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Shanker G, Syversen T, Aschner JL, Aschner M. Modulatory effect of glutathione status and antioxidants on methylmercury-induced free radical formation in primary cultures of cerebral astrocytes. Brain Res Mol Brain Res. 2005;137:11-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 100] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 6. | Khanum F, Anilakumar KR, Viswanathan KR. Anticarcinogenic properties of garlic: a review. Crit Rev Food Sci Nutr. 2004;44:479-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 101] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 7. | Banerjee SK, Maulik M, Manchanda SC, Dinda AK, Das TK, Maulik SK. Garlic-induced alteration in rat liver and kidney morphology and associated changes in endogenous antioxidant status. Food Chem Toxicol. 2001;39:793-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Pal R, Vaiphei K, Singh K, Rana SV. Garlic confers hepatoprotection in isoniazid rifampicin induced hepatic injury. Ind J Gastro. 2003;22:A100. |
| 9. | Bahri AK, Chiang CS, Timbrell JA. Acetylhydrazine hepatotoxicity. Toxicol Appl Pharmacol. 1981;60:561-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Wang EJ, Li Y, Lin M, Chen L, Stein AP, Reuhl KR, Yang CS. Protective effects of garlic and related organosulfur compounds on acetaminophen-induced hepatotoxicity in mice. Toxicol Appl Pharmacol. 1996;136:146-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 66] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Kornbrust DJ, Mavis RD. The effect of paraquat on microsomal lipid peroxidation in vitro and in vivo. Toxicol Appl Pharmacol. 1980;53:323-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 59] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal Biochem. 1968;25:192-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5089] [Cited by in RCA: 5394] [Article Influence: 94.6] [Reference Citation Analysis (0)] |
| 13. | Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265-275. [PubMed] |
| 14. | Lawson LD, Wang ZJ. Allicin and allicin-derived garlic compounds increase breath acetone through allyl methyl sulfide: use in measuring allicin bioavailability. J Agric Food Chem. 2005;53:1974-1983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 105] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 15. | Amagase H, Petesch BL, Matsuura H, Kasuga S, Itakura Y. Intake of garlic and its bioactive components. J Nutr. 2001;131:955S-962S. [PubMed] |
| 16. | Reuter HD, Koch HP, Lowson DL; Therapeutic effects and applications of garlic and its preparation. In: Garlic: The science and therapeutic application of Allium sativum L and related species. 2nd ed. (Koch, HP and Lawson, DL. eds). William and Wilkins, Baltimore MB, pp 135 - 212. . |
| 17. | Hodge G, Hodge S, Han P. Allium sativum (garlic) suppresses leukocyte inflammatory cytokine production in vitro: potential therapeutic use in the treatment of inflammatory bowel disease. Cytometry. 2002;48:209-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 134] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 18. | Park EY, Ki SH, Ko MS, Kim CW, Lee MH, Lee YS, Kim SG. Garlic oil and DDB, comprised in a pharmaceutical composition for the treatment of patients with viral hepatitis, prevents acute liver injuries potentiated by glutathione deficiency in rats. Chem Biol Interact. 2005;155:82-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Bruck R, Aeed H, Brazovsky E, Noor T, Hershkoviz R. Allicin, the active component of garlic, prevents immune-mediated, concanavalin A-induced hepatic injury in mice. Liver Int. 2005;25:613-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | MacDonald JA, Marchand ME, Langler RF. Improving upon the in vitro biological activity of antithrombotic disulfides. Blood Coagul Fibrinolysis. 2004;15:447-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Munday R, Munday CM. Induction of phase II enzymes by aliphatic sulfides derived from garlic and onions: an overview. Methods Enzymol. 2004;382:449-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Oz HS, McClain CJ, Nagasawa HT, Ray MB, de Villiers WJ, Chen TS. Diverse antioxidants protect against acetaminophen hepatotoxicity. J Biochem Mol Toxicol. 2004;18:361-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 80] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 23. | Kalantari H, Salehi M. The protective effect of garlic oil on hepatotoxicity induced by acetaminophen in mice and comparison with N-acetylcysteine. Saudi Med J. 2001;22:1080-1084. [PubMed] |
| 24. | Tanaka S, Haruma K, Kunihiro M, Nagata S, Kitadai Y, Manabe N, Sumii M, Yoshihara M, Kajiyama G, Chayama K. Effects of aged garlic extract (AGE) on colorectal adenomas: a double-blinded study. Hiroshima J Med Sci. 2004;53:39-45. [PubMed] |