Published online Jan 28, 2006. doi: 10.3748/wjg.v12.i4.625
Revised: April 28, 2005
Accepted: May 24, 2005
Published online: January 28, 2006
AIM: To investigate the occurrence of chromosome 3, 7, 8, 9, and 17 aneuploidies, TP53 gene deletion and p53 protein expression in chronic gastritis, atrophic gastritis and gastric ulcer, and their association with H pylori infection.
METHODS: Gastric biopsies from normal mucosa (NM, n = 10), chronic gastritis (CG, n = 38), atrophic gastritis (CAG, n=13) and gastric ulcer (GU, n = 21) were studied using fluorescence in situ hybridization (FISH) and immunohistochemical assay. A modified Giemsa staining technique and PCR were used to detect H pylori. An association of the gastric pathologies and aneuploidies with H pylori infection was assessed.
RESULTS: Aneuploidies were increasingly found from CG (21%) to CAG (31%) and to GU (62%), involving mainly monosomy and trisomy 7, trisomies 7 and 8, and trisomies 7, 8 and 17, respectively. A significant association was found between H pylori infection and aneuploidies in CAG (P = 0.0143) and GU (P = 0.0498). No TP53 deletion was found in these gastric lesions, but p53-positive immunoreactivity was detected in 45% (5/11) and 12% (2/17) of CG and GU cases, respectively. However, there was no significant association between p53 expression and H pylori infection.
CONCLUSION: The occurrence of aneuploidies in benign lesions evidences chromosomal instability in early stages of gastric carcinogenesis associated with H pylori infection, which may confer proliferative advantage. The increase of p53 protein expression in CG and GU may be due to overproduction of the wild-type protein related to an inflammatory response in mucosa.
- Citation: César ACG, Calmon MF, Cury PM, Caetano A, Borim AA, Silva AE. Genetic alterations in benign lesions: Chronic gastritis and gastric ulcer. World J Gastroenterol 2006; 12(4): 625-629
- URL: https://www.wjgnet.com/1007-9327/full/v12/i4/625.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i4.625
Gastric carcinogenesis is considered a multi-step and multi-factorial process that proceeds from normal gastric mucosa with epithelial hyperproliferation to chronic gastritis with variable degrees of atrophy, intestinal metaplasia, dysplasia, and ultimately carcinoma[1]. In most cases, chronic gastritis, frequently associated with H pylori infection, precedes the formation of gastric cancer, and a great proportion of the clinical tumors occurs in connection with severe atrophic gastritis and extensive intestinal metaplasia[2]. Efforts have been made to identify changes of a precancerous nature in the gastric epithelium. The identification of such changes may help to prevent the occurrence of gastric cancer[3].
Gastritis may also play a role in the pathogenesis of peptic ulcer disease by impairing the resistance of the gastric mucosa and consequently increasing the risk of ulcer[3]. In practice, the cancer risk in peptic ulcer disease applies exclusively to gastric ulcer. Epidemiological data on the incidence of peptic ulcer disease with gastric cancer suggest that ulcer disease may not be an important precursor of gastric cancer. However, possible links between these two are suggested by a similar relationship with chronic gastritis[2].
H pylori infection is accepted as the major causal factor for the histological features leading to severe gastroduodenal disease, including chronic gastritis, peptic ulcer disease, intestinal-type gastric cancer and gastric MALT lymphoma. Only a small proportion of the infected cases will develop clinically significant disease. About one in 5-6 individuals will develop peptic ulcer disease during their lifetime, and <1% will develop gastric cancer[4].
It is particularly important to recognize and define the characteristics of benign and pre-malignant lesions for application to studies of carcinogenesis and cancer prevention. Hence, in this study, we aimed to verify the occurrence of aneuploidies of chromosomes 3, 7, 8, 9, and 17, and of TP53 gene deletions using the FISH technique, besides p53 expression, in benign lesions such as chronic gastritis, atrophic gastritis and gastric ulcer. The results were associated with H pylori infection and other clinicopathological features, such as sex, age, smoking and alcohol consumption.
Chronic gastritis (CG), atrophic gastritis (CAG) and gastric ulcer (GU) samples were obtained, respectively, from 38 (13 males and 25 females), 13 (7 males and 6 females) and 21 patients (15 males and 6 females) without gastric cancer by biopsy performed during endoscopic evaluation. The mean age of these patients was 53 years (range, 2-89 years). Biopsies of histologically confirmed normal and H pylori-negative mucosa (NM) were obtained from 10 healthy individuals (2 males and 8 females) with a mean age of 43 years (range, 19-75 years). All specimens were collected at the Hospital de Base of São José do Rio Preto, SP, Brazil. All biopsies of normal mucosa and gastric ulcer were collected from the antral region of the stomach, whereas those of chronic gastritis and atrophic gastritis were mainly collected from the antrum and corpus. Clinicopathological data were collected using a standard interviewer-administered questionnaire and a review of the patients’ medical records. The study was approved by the National Research Ethics Committee, and written informed consent was obtained from all subjects.
Hematoxylin-eosin (H&E) staining was used for the diagnosis and classification of gastritis[5]. A modified Giemsa staining technique was used to visualize H pylori.
DNA was extracted by the phenol-chloroform method after digestion with proteinase K[6]. PCR assays were performed separately with approximately 100 ng of total DNA, using three different sets of oligonucleotides. One of them amplifies a 312-bp segment of the gene CYP1A[7] as a DNA quality control, the other amplifies a 298-bp product of the gene encoding species-specific H pylori antigen[8], and the last one amplifies a 411-bp fragment corresponding to the urease A gene[9]. Positive and negative controls were used in all experiments. PCR products were separated on 75 g/L polyacrylamide-gel electrophoresis, followed by silver nitrate staining. The assay was considered positive when at least one of the bacterial PCR products was present[10].
Nuclear suspensions from all samples were obtained by mechanical and enzymatic disaggregation[11]. After washes in 600 mL/L acetic acid, the resulting nuclear suspension was dropped onto microscope slides and stored at -70 °C until use for FISH assay.
FISH analysis was performed on interphase nuclei using centromere probes (CEP) for chromosomes 3, 7, and 8 directly labeled with Spectrum Green, and for chromosome 9 labeled with rhodamine (Vysis, Inc., Downers Grove, IL, USA). Dual-color FISH assays were performed using TP53 probe kits (Vysis), including the LSI TP53 sequence labeled in Spectrum Orange, with the centromere probe for chromosome 17 labeled in Spectrum Green. The hybridization and detection protocols followed the manufacturer’s instructions.
For each probe, signals of about 300 intact and non-overlapping nuclei were evaluated by two independent observers according to the criteria described by Eastmond et al[12]. Cutoff levels for aneuploidies were based on the upper-limit mean +4 SD of the normal gastric mucosa biopsies. By definition, for monosomy of chromosomes 3, 7, 8, 9, and 17, the cutoff values were set at 10.0%, 11.0%, 11.0%, 8.5%, and 7.5%, respectively, and, for trisomy of the same chromosomes, at 10.0%, 9.0%, 8.0%, 8.5%, and 7.0%, respectively. Tetrasomy and pentasomy were absent in the normal mucosa samples; thus, 2% was arbitrarily taken as cutoff value.
The TP53 gene scores were taken together with those of the chromosome 17 centromere. For TP53 gene deletion analysis, the gene-to-centromere ratio (G/C) was calculated for each case, that is, the total number of gene signals observed was divided by the total number of centromere signals.
To assess p53 protein accumulation, the commercially available monoclonal antibody DO-7 (Novocastra, Newcastle, UK) was used in combination with the anti-Ig second-stage antibody and the ABC kit (Novocastra) on paraffin-embedded sections. This antibody recognizes both mutant and wild types of p53 protein. The slides were counterstained with Harris hematoxylin. As positive controls, sections from gastric adenocarcinoma were processed, previously shown to express high levels of p53 protein. For each sample, 500 epithelial cells were counted.
Data were analyzed using χ2 and Fisher’s exact tests. P values less than 0.05 were considered statistically significant.
Analysis by the FISH technique showed that 89% to 99% of the nuclei of the H pylori-negative normal mucosa cases were disomic for chromosomes 3, 7, 8, 9, and 17, so they were considered to have no aneuploidy. The gene-to-centromere ratio (G/C) for the TP53 gene deletion ranged from 0.97 to 0.99 (mean of 0.98), so, in the cases of benign lesions with a G/C ratio < 0.90, gene deletion was assumed. Low-intensity nuclear p53 protein immunoreactivity was observed only in 4 of the 8 evaluated cases of normal gastric mucosa, ranging from 0.2% to 4.0% of the immunostained nuclei. The positivity index was defined as >10% of cells showing nuclear staining, according to a previous report by Gobbo-César et al[11].
Table 1 shows the results regarding aneuploidies of chromosomes 3, 7, 8, 9, and 17, TP53 gene deletion, p53 expression, and clinicopathological data only of those cases with benign lesions (chronic gastritis, atrophic gastritis and gastric ulcer) which showed some genetic alteration.
| Cases | Sex/age | Smoking/alcoholconsumption | H pylori | L | TP53deletion(G/C<0.9) | p53IHC (>10%) | Aneuploidy |
| Chronic gastritis | |||||||
| CG03 | M/32 | No/No | - | A | ND | ND | -7, +8a |
| CG06 | M/02 | No/No | - | A | 0.97 | 15.6 | |
| CG13 | F/56 | Yes/No | + | C | 0.95 | 31.4 | |
| CG14 | F/36 | Yes/No | + | A | 0.94 | 0 | -7 |
| CG16 | M/89 | Yes/Yes | + | C | 0.98 | 12.0 | |
| CG19 | F/54 | No/Yes | - | A | 0.90 | 15.0 | +7 |
| CG30 | F/41 | Yes/No | + | A | ND | ND | +7a |
| CG31 | M/44 | Yes/No | + | A | 0.95 | ND | +9 |
| CG33 | F/57 | No/No | + | A | 0.96 | 12.8 | |
| CG34 | F/34 | Yes/Yes | + | A | ND | ND | +7 |
| CG35 | M/54 | Yes/Yes | + | A | ND | ND | +3, +7, +17 |
| CG36 | F/58 | No/No | + | A | 0.96 | 0.8 | -7 |
| Atrophic gastritis | |||||||
| CAG05 | F/84 | No/No | + | A | ND | ND | +8a |
| CAG07 | M/65 | Yes/Yes | + | A | ND | ND | +7, +8 |
| CAG08 | M/57 | No/Yes | + | A | ND | ND | +7 / +7+7 |
| CAG13 | M/77 | Yes/No | + | A | ND | ND | +9 |
| Gastric ulcer | |||||||
| GU02 | M/73 | Yes/Yes | - | A | ND | 0.8 | +17 |
| GU03 | M/52 | Yes/Yes | - | A | ND | 47.2 | +9 / +9+9 |
| GU04 | M/78 | Yes/Yes | - | A | ND | 9.6 | +7, +8a |
| GU05 | M/64 | Yes/Yes | - | A | ND | 0.8 | -7a |
| GU06 | M/43 | Yes/No | + | A | ND | 0 | +7 / +7+7, +8, –9, +17 / +17+17 |
| GU07 | F/51 | Yes/Yes | + | A | ND | 1.2 | +3 / +3+3 |
| GU09 | F/72 | No/No | + | A | 0.97 | 14.6 | |
| GU12 | M/28 | Yes/Yes | + | A | ND | 3.2 | -7, -9, -17 |
| GU14 | F/83 | No/No | + | A | ND | 1.2 | -9 |
| GU15 | M/43 | Yes/Yes | + | A | ND | 6.4 | -7, -8 |
| GU16 | F/82 | No/No | + | A | ND | 0 | -8 |
| GU18 | M/58 | Yes/Yes | + | A | ND | ND | +7 / +7+7 |
| GU19 | F/62 | Yes/No | + | A | ND | ND | +7 / +7+7, +8, +17 / +17+17 |
| GU20 | M/63 | Yes/Yes | + | A | ND | ND | +9 / +9+9a |
Increasing frequencies of aneuploidies were detected in the CG, CAG and GU cases: 21% (8/38), 31% (4/13), and 62% (13/21), respectively. The samples also showed an increasing complexity of the alterations with mainly monosomy and trisomy of chromosome 7 in CG, trisomy of chromosomes 7 and 8 in CAG, and more frequent trisomy of chromosomes 7, 8 and 17 in GU. About 75% (6/8) of CG, 100% (4/4) of CAG and 69% (9/13) of GU with aneuploidies were H pylori-positive. However, a significant association between the presence of aneuploidies and H pylori infection was found only in CAG (P = 0.0143) and GU (P = 0.0498), while parameters such as sex, age, smoking and alcohol consumption did not show any association with the occurrence of aneuploidies.
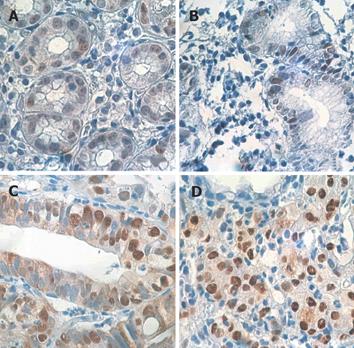
No TP53 gene deletion was found in the cases evaluated, whether in CG, in CAG or in GU, but low-intensity nuclear p53-positive immunoreactivity was present in 45% (5/11) and 12% (2/17) of the CG and GU cases, respectively, ranging from 12% to 47.2% of p53-positive nuclei, as compared to over-expression of the p53 protein in positive controls from gastric adenocarcinoma (Figure 1). No significant association was found either between p53 expression and H pylori infection or with the other clinicopathological features investigated.
The development of gastric carcinoma is considered as a multistage progressive process due to alterations in oncogenes, tumor suppressor genes, cell-adhesion molecules, telomerase, as well as to a genetic instability at several microsatellite loci, possibly related to H pylori infection[13,14]. The mechanisms leading to mutations, hyperproliferation and apoptosis of the epithelial cells are triggered very early in the H pylori gastritis cascade, resulting in atrophic gastritis, intestinal metaplasia and gastric cancer[2,13].
The identification of genetic alterations in biopsies of the epithelium of patients with gastric dyspepsia may be helpful in early diagnosis of gastric cancer. FISH is an accurate and sensitive technique for the detection of aneuploidies in interphase nuclei from biopsies, making it possible to identify changes in early stages of benign lesions, such as gastritis and gastric ulcer. Such changes may be used as markers for estimating the risk of gastric cancer.
A few studies have evaluated aneuploidy in epithelial lesions of the stomach using DNA flow cytometry. In chronic and atrophic gastritis, Weiss et al[15] found DNA aneuploidy in 5.2% and in 8.5% of the cases, respectively. Teodori et al[16] observed aneuploidy in 45% (9/20) chronic atrophic gastritis specimens, whereas Abdel-Wahab et al[3] detected quantitative DNA alterations in 18.8% of atrophic gastritis cases.
To our knowledge, studies about aneuploidy in gastric ulcer are not available in the literature. Our study using the FISH technique showed increasing aneuploidy and complexity of the alterations from chronic gastritis (21%) towards atrophic gastritis (31%) and gastric ulcer (62%). The more frequent aneuploidies were monosomy and trisomy 7 in CG, trisomies 7 and 8 in CAG, and trisomies 7, 8, and 17 in GU. Moreover, in CAG and GU, these aneuploidies occurred more frequently in the H pylori-positive cases, probably being responsible for the chromosomal instability observed.
Chromosomes 7, 8 and 17 carry several important genes involved in the control of cell proliferation, such as EGFR (7p12-13), c-myc (8q24), HER-2/neu (17q21), and TP53 (17p13). Therefore, the presence of trisomy of these chromosomes in precancerous gastric lesions leading to gene imbalance may confer proliferative advantage, increasing the risk of developing into gastric cancer.
The development of carcinoma in cases of gastric ulcer disease during long-term H2-blocker treatment is slowly increasing, and ulcers that require such treatment exhibit the characteristics of intractable conditions, including linear ulcers, simultaneous gastric and duodenal ulcers, immature intestinal metaplasia of the gastric epithelium, and atrophic gastritis accompanied by multiple ulcer cicatrices[17]. Hence, especially in these cases of gastric ulcer, it is suggested that a genetic study should be performed to allow early detection of malignant transformation.
Inactivation of the TP53 gene by mutation or deletion occurs as an initial event in gastric tumorigenesis, possibly associated with H pylori infection[14,18,19]. Using the FISH technique, Gomyo et al[20] detected a TP53 deletion in 77% (10/13) cases of gastric carcinoma.
In the present study, no TP53 deletion was found in either chronic gastritis (15 cases), atrophic gastritis (3 cases) or gastric ulcer (3 cases), although it was observed in 60% (3/5) of the intestinal metaplasia and 110% (3/3) of the adenocarcinoma cases in a previous study conducted by our group[11], thereby corroborating the hypothesis that these pathologies share genetic alterations and progress jointly.
The p53 gene products are known to regulate cell growth and proliferation. Wild-type p53 protein suppresses cell growth by controlling the G1 checkpoint, and has several additional physiological functions, including control of the G2 cell checkpoint, as well as mediation of apoptosis[21]. The products of wild-type p53 can hardly be detected in normal cells by immunohistochemical assays because of their short half-life. However, TP53 gene alterations induced by many missense mutations may modify the conformation and stabilize the proteins, leading to their accumulation[22]. Therefore, over-expression of a p53 protein is a useful marker for detecting a mutated TP53 gene[23,24].
In this study, an increase of detectable low-intensity p53 was observed in 45% (5/11) of chronic gastritis and 12% (2/17) of gastric ulcer cases. These positive immunostaining findings may be a consequence of point mutations or small deletions. However, these changes are not detected by FISH, so were not tested in the current study. Alternatively, such alterations are more likely to be due to over-expression of the wild-type protein related to a physiological response brought about by the inflammatory process of the gastric epithelium. The lack of association between p53 expression and H pylori infection may be related to the reduced number of specimens from benign lesions evaluated.
Although some studies have described p53 protein accumulation in benign gastric lesions[13,25-28], its association with H pylori infection in gastric carcinogenesis is not yet clear. Whereas some authors reported over-expression of p53 protein in H pylori-positive chronic gastritis and intestinal metaplasia[13,25,27,28], Craanen et al[29] did not observe p53 protein accumulation in either atrophic gastritis or intestinal metaplasia and suggested that this process may be a late event in gastric carcinogenesis. On the other hand, Lan et al[13] reported that H pylori infection might first cause severe imbalance of proliferation and apoptosis in the precancerous lesions, leading to p53-Rb tumor suppressor system mutation and telomerase reactivation, and finally causing gastric cancer.
In conclusion, our study has clearly shown the occurrence of aneuploidies, which increases with the progression of lesions from chronic gastritis to atrophic gastritis and to gastric ulcer, is frequently associated with H pylori infection, evidencing chromosomal instability in benign gastric lesions that may play an important role in gastric carcinogenesis. We observed no TP53 gene deletion in the benign gastric lesions, whereas p53 protein over-expression was detected in some cases of chronic gastritis and gastric ulcer, thus suggesting that an accumulation of wild-type p53 protein occurs, probably due to an inflammatory response.
This work was accomplished in the Cytogenetics and Molecular Biology Laboratory, Department of Biology, UNESP - São Paulo State University, São José do Rio Preto, SP, Brazil
S- Editor Kumar M and Wang J L- Editor Elsevier HK E- Editor Bi L
| 1. | Correa P. Human gastric carcinogenesis: a multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52:6735-6740. [PubMed] |
| 2. | Sipponen P, Hyvärinen H, Seppälä K, Blaser MJ. Review article: Pathogenesis of the transformation from gastritis to malignancy. Aliment Pharmacol Ther. 1998;12 Suppl 1:61-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 66] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 3. | Abdel-Wahab M, Attallah AM, Elshal MF, Eldousoky I, Zalata KR, el-Ghawalby NA, Gad el-Hak N, el-Ebidy G, Ezzat F. Correlation between endoscopy, histopathology, and DNA flow cytometry in patients with gastric dyspepsia. Hepatogastroenterology. 1996;43:1313-1320. [PubMed] |
| 4. | Go MF. Review article: natural history and epidemiology of Helicobacter pylori infection. Aliment Pharmacol Ther. 2002;16 Suppl 1:3-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 275] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 5. | Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3221] [Cited by in RCA: 3550] [Article Influence: 122.4] [Reference Citation Analysis (3)] |
| 6. | Sambrook J, Russel , DW . Molecular Cloning: A Laboratory Manual. 3rd ed. New York: Cold Spring Harbor Lab Press 2001; 6.4-6.11. |
| 7. | Abdel-Rahman SZ, el-Zein RA, Anwar WA, Au WW. A multiplex PCR procedure for polymorphic analysis of GSTM1 and GSTT1 genes in population studies. Cancer Lett. 1996;107:229-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 198] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 8. | Hammar M, Tyszkiewicz T, Wadström T, O'Toole PW. Rapid detection of Helicobacter pylori in gastric biopsy material by polymerase chain reaction. J Clin Microbiol. 1992;30:54-58. [PubMed] |
| 9. | Clayton CL, Kleanthous H, Coates PJ, Morgan DD, Tabaqchali S. Sensitive detection of Helicobacter pylori by using polymerase chain reaction. J Clin Microbiol. 1992;30:192-200. [PubMed] |
| 10. | Dzierzanowska D, Gzyl A, Rozynek E, Augustynowicz E, Wojda U, Celińska-Cedro D, Sankowska M, Wadström T. PCR for identification and typing of Helicobacter pylori isolated from children. J Physiol Pharmacol. 1996;47:101-114. [PubMed] |
| 11. | César AC, Borim AA, Caetano A, Cury PM, Silva AE. Aneuploidies, deletion, and overexpression of TP53 gene in intestinal metaplasia of patients without gastric cancer. Cancer Genet Cytogenet. 2004;153:127-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Eastmond DA, Schuler M, Rupa DS. Advantages and limitations of using fluorescence in situ hybridization for the detection of aneuploidy in interphase human cells. Mutat Res. 1995;348:153-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 59] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Lan J, Xiong YY, Lin YX, Wang BC, Gong LL, Xu HS, Guo GS. Helicobacter pylori infection generated gastric cancer through p53-Rb tumor-suppressor system mutation and telomerase reactivation. World J Gastroenterol. 2003;9:54-58. [PubMed] |
| 14. | Tahara E. Genetic pathways of two types of gastric cancer. IARC Sci Publ. 2004;327-349. [PubMed] |
| 15. | Weiss H, Gütz HJ, Schröter J, Wildner GP. DNA distribution pattern in chronic gastritis. I. DNA ploidy and cell cycle distribution. Scand J Gastroenterol. 1989;24:643-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Teodori L, Capurso L, Cordelli E, De Vita R, Koch M, Tarquini M, Pallone F, Mauro F. Cytometrically determined relative DNA content as an indicator of neoplasia in gastric lesions. Cytometry. 1984;5:63-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 42] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Aoki T, Takayama S, Nimura H, Tsutsumi J. Effects of medical treatment on gastric mucosal abnormalities in gastroduodenal ulcer disease. World J Surg. 2000;24:321-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 18. | Murakami K, Fujioka T, Okimoto T, Mitsuishi Y, Oda T, Nishizono A, Nasu M. Analysis of p53 gene mutations in Helicobacter pylori-associated gastritis mucosa in endoscopic biopsy specimens. Scand J Gastroenterol. 1999;34:474-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Wang J, Chi DS, Kalin GB, Sosinski C, Miller LE, Burja I, Thomas E. Helicobacter pylori infection and oncogene expressions in gastric carcinoma and its precursor lesions. Dig Dis Sci. 2002;47:107-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Gomyo Y, Osaki M, Kaibara N, Ito H. Numerical aberration and point mutation of p53 gene in human gastric intestinal metaplasia and well-differentiated adenocarcinoma: analysis by fluorescence in situ hybridization (FISH) and PCR-SSCP. Int J Cancer. 1996;66:594-599. [PubMed] |
| 21. | Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5482] [Cited by in RCA: 5460] [Article Influence: 195.0] [Reference Citation Analysis (0)] |
| 22. | Finlay CA, Hinds PW, Tan TH, Eliyahu D, Oren M, Levine AJ. Activating mutations for transformation by p53 produce a gene product that forms an hsc70-p53 complex with an altered half-life. Mol Cell Biol. 1988;8:531-539. [PubMed] |
| 23. | Rodrigues NR, Rowan A, Smith ME, Kerr IB, Bodmer WF, Gannon JV, Lane DP. p53 mutations in colorectal cancer. Proc Natl Acad Sci U S A. 1990;87:7555-7559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 629] [Cited by in RCA: 711] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 24. | Chang F, Syrjänen S, Kurvinen K, Syrjänen K. The p53 tumor suppressor gene as a common cellular target in human carcinogenesis. Am J Gastroenterol. 1993;88:174-186. [PubMed] |
| 25. | Marinone C, Martinetti A, Mestriner M, Seregni E, Geuna M, Ferrari L, Strola G, Bonardi L, Fea E, Bombardieri E. p53 evaluation in gastric mucosa of patients with chronic Helicobacter pylori infection. Anticancer Res. 2001;21:1115-1118. [PubMed] |
| 26. | Feng CW, Wang LD, Jiao LH, Liu B, Zheng S, Xie XJ. Expression of p53, inducible nitric oxide synthase and vascular endothelial growth factor in gastric precancerous and cancerous lesions: correlation with clinical features. BMC Cancer. 2002;2:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 98] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 27. | Unger Z, Molnár B, Prónai L, Szaleczky E, Zágoni T, Tulassay Z. Mutant p53 expression and apoptotic activity of Helicobacter pylori positive and negative gastritis in correlation with the presence of intestinal metaplasia. Eur J Gastroenterol Hepatol. 2003;15:389-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Jorge O, Cuello Carrión FD, Jorge A, Ciocca DR. Helicobacter pylori infection affects the expression of PCNA, p53, c-erbB-2 and Bcl-2 in the human gastric mucosa. Rev Esp Enferm Dig. 2003;95:97-104, 89-96. [PubMed] |
| 29. | Craanen ME, Blok P, Dekker W, Offerhaus GJ, Tytgat GN. Chronology of p53 protein accumulation in gastric carcinogenesis. Gut. 1995;36:848-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 1.7] [Reference Citation Analysis (0)] |