Published online Jan 28, 2006. doi: 10.3748/wjg.v12.i4.582
Revised: July 15, 2005
Accepted: August 3, 2005
Published online: January 28, 2006
AIM: To investigate distal small bowel motility and lipid absorption in patients following elective abdominal aortic aneurysm (AAA) repair surgery.
METHODS: Nine patients (aged 35-78 years; body mass index (BMI) range: 23-36 kg/m2) post-surgery for AAA repair, and seven healthy control subjects (20-50 years; BMI range: 21-29 kg/m2) were studied. Continuous distal small bowel manometry was performed for up to 72 h, during periods of fasting and enteral feeding (Nutrison®). Recordings were analyzed for the frequency, origin, length of migration, and direction of small intestinal burst activity. Lipid absorption was assessed on the first day and the third day post surgery in a subset of patients using the 13C-triolein-breath test, and compared with healthy controls. Subjects received a 20-min intraduodenal infusion of 50 mL liquid feed mixed with 200 μL 13C-triolein. End-expiratory breath samples were collected for 6 h and analyzed for 13CO2 concentration.
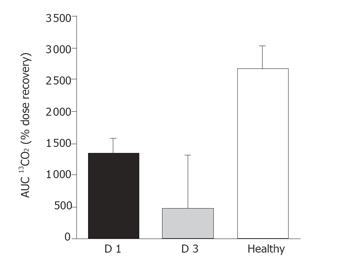
RESULTS: The frequency of burst activity in the proximal and distal small intestine was higher in patients than in healthy subjects, under both fasting and fed conditions (P < 0.005). In patients there was a higher proportion of abnormally propagated bursts (71% abnormal), which began to normalize by d 3 (25% abnormal) post-surgery. Lipid absorption data was available for seven patients on d 1 and four patients on d 3 post surgery. In patients, absorption on d 1 post-surgery was half that of healthy control subjects (AUC 13CO2 1 323 ± 244 vs 2 646 ±365; P < 0.05, respectively), and was reduced to the one-fifth that of healthy controls by d 3 (AUC 13CO2 470 ± 832 vs 2 646 ± 365; P < 0.05, respectively).
CONCLUSION: Both proximal and distal small intestinal motor activity are transiently disrupted in critically ill patients immediately after major surgery, with abnormal motility patterns extending as far as the ileum. These motor disturbances may contribute to impaired absorption of enteral nutrition, especially when intraluminal processing is necessary for efficient digestion.
- Citation: Fraser RJ, Ritz M, Matteo ACD, Vozzo R, Kwiatek M, Foreman R, Stanley B, Walsh J, Burnett J, Jury P, Dent J. Distal small bowel motility and lipid absorption in patients following abdominal aortic aneurysm repair surgery. World J Gastroenterol 2006; 12(4): 582-587
- URL: https://www.wjgnet.com/1007-9327/full/v12/i4/582.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i4.582
Enteral feeding reduces both morbidity and mortality in critically ill patients[1]. A number of factors, including high gastric aspirate volumes[1,2], reduce the effectiveness of enteral feeding in critically ill patients by delaying its commencement or continuation. Slow gastric emptying is a major cause of difficulties with intragastric delivery of feeds, and occurs in 30-50% of mechanically ventilated patients[3]. Prokinetic stimulation of gastric motility or bypassing the stomach with jejunal feeding tubes overcomes some of these difficulties[4]. However, gastrointestinal symptoms such as diarrhea, abdominal distention, vomiting, and regurgitation remain common in enterally fed, critically ill patients[2]. The motor mechanisms responsible for these symptoms are poorly understood, largely because data on small intestinal motility in critically ill patients are very limited.
Previous studies have shown that the patterning of interdigestive motor activity in the proximal small intestine is frequently disturbed in the critically ill. Bursts of abnormally propagated pressure waves that resemble phase III of the migrating motor complex are present, and persist during enteral feeding[5-7]. The potential impact of this motor disturbance on nutrient absorption in the critically ill has not been assessed. We have previously shown in healthy human beings that lipid absorption is reduced when phase III activity is stimulated during enteral feeding[8]. Previous manometric studies in critically ill patients have been limited to the proximal and mid small intestine[5-7]. It is unknown whether the motor changes observed proximally are present further down the gut. If such abnormalities prevail along the full length of the intestine, they are likely to have a greater negative impact on the success of enteral feeding.
In this study, we have recorded intraluminal pressure patterns along the distal small intestine in a group of patients immediately after abdominal aortic aneurysm (AAA) repair surgery, and for the following 3 d. In a subset of patients, these findings were correlated with the measurements of lipid absorption using the 13C-triolein breath test[9].
Nine patients (35-78 years: body mass index (BMI) range 23-36 kg/m2: APACHE II score[10] range 6-33) participated in the study following elective AAA repair surgery at Saint Andrew’s Hospital, Adelaide during their post-surgical admission to the intensive care unit (ICU). All patients gave written informed consent before their surgery. Patients who had previously undergone upper gastrointestinal tract surgery or who were on medication known to affect gastrointestinal motility (other than the usual post-surgical drugs e.g. opiate analgesics) were excluded from the study.
Seven healthy control subjects (20-50 years: BMI range 21-29 kg/m2) were studied. Individuals with a history of cardiovascular, respiratory or gastrointestinal disease including severe anemia, eating disorders, diabetes mellitus or other serious past medical history were excluded. Subjects were not permitted to use medication (apart from paracetamol) in the two weeks before the study. Subjects gave written informed consent prior to entering the study.
The protocol was approved by the human research ethics committees of Saint Andrew’s Hospital and the Repatriation General Hospital South Australia.
In all the subjects, small intestinal pressure waves were measured using a 200-cm-long, purpose-built silicone rubber multilumen assembly (outer diameter 4 mm) (Dentsleeve, Adelaide, South Australia), incorporating 16 pressure recording channels (inner diameter 0.4 mm). The two most proximal side-holes were spaced 5 cm apart and the following 14 at 12.5-cm intervals. A single larger channel of the assembly (inner diameter 0.9 mm, located 142 cm from the distal tip) was used to deliver enteral feeds into the distal duodenum. Correct positioning of the assembly was facilitated by small weights and a balloon (7 mL) located at the tip of the assembly.
All manometric lumina were perfused with degassed distilled water at a constant rate of 0.05 mL/min. This resulted in a total daily infusion volume of 1 150 mL/d of water, which was accounted for in the patients’ daily fluid, electrolyte, and nutrient requirements. Pressures were measured via external transducers (Abbott Critical Care Systems, North Chicago, IL, USA) and recorded onto a Power Macintosh G3 computer using MPS 100 software (Biopac System Inc., Santa Barbara, CA, USA).
Patients were studied continuously over 3 d immediately following surgical repair of AAA. The manometric assembly was passed transnasally and its tip positioned in the stomach prior to surgery. The assembly incorporated a silicone rubber balloon at its distal tip that when inflated allowed the surgeon to palpate the tip of the catheter in the stomach. At the time of surgery, the assembly was passed through the pylorus, along the duodenum, jejunum, and ileum by manipulation of the inflated balloon by the surgeon, and by intrinsic motor activity. The final position of the assembly was determined by routine abdominal X-ray.
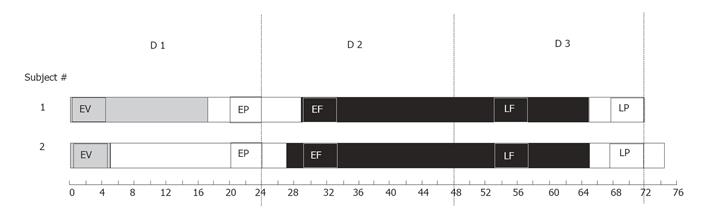
Measurement of intraluminal pressures commenced immediately after the arrival of patients in the ICU and continued for approximately 72 h. Patients were fasted for the first 24 h post surgery (d 1), during which low-pressure suction was used to aspirate gastric contents. On d 2, enteral feeding (Nutrison®, Nutrica, Zoetermeer, Holland; 40-80 mL/h) was commenced via the infusion channel incorporated into the manometric assembly. The effects of enteral feeding on small intestinal motility were assessed in two time windows: (1) immediately after commencing enteral feeding (d 2; early feeding period, n = 8) and (2) over the following 24 h (d 3; late feeding period, n = 8). These data were averaged to obtain final feeding data (Table 1).
| Description | t(postsurgery)/h | Feedingcondition | Ventilationcondition | n |
| Early ventilation | 1–5 | No enteral feeding | Ventilation | 9 |
| Early post-operative | 20–24 | No enteral feeding | No ventilation | 7 |
| Early feeding | 29–33 | Enteral feeding | No ventilation | 8 |
| Late feeding | 53–57 | Enteral feeding | No ventilation | 8 |
| Late post-operative | 68–72 | No enteral feeding | No ventilation | 9 |
Healthy subjects arrived at the Repatriation General Hospital Gastrointestinal Ward after an overnight fast from 2 000 h (except for water). The manometric assembly was introduced into the stomach via an anesthetized nostril (Lignocaine 50 g/L, PaedPharm, Australia). The subject was then positioned in the right lateral position until the tip of the assembly had passed beyond the pylorus. Progression of the assembly along the small intestine was assisted by inflation of the catheter balloon. Correct positioning of the assembly occurred when only the two most proximal side-holes remained in the gastric antrum, which was verified by the analysis of pressure wave frequencies[13]. Side holes located within the first 25 cm of the small intestine were considered to be in the duodenum, side holes in the next 120 cm were defined to be in the jejunum, and the remaining side holes were considered to be in the ileum.
Manometric recordings commenced immediately following the correct placement of the assembly and continued for 72 h. Pressures were measured during fasting (0-24 h) and enteral feeding (Nutrison®, Nutrica, Zoetermeer, Holland; 80 mL/h) (24-72 h).
Lipid absorption was assessed using the 13C-triolein breath test[9]. In patients, absorption was evaluated on d 1 (n = 7) and d 3 (n = 4) post surgery. Absorption data from both days were compared to healthy subjects (n = 7). After a minimum of 4 h fasting, 50 mL of a mixed nutrient liquid (Nutrison®, Nutrica, Zoetermeer, Holland) was mixed with 200 µL of [1,1,1-13C3] triolein (99%, 200 mg) (Cambridge Isotope Laboratories, Andover, MA, USA) and infused intraduodenally over 20 min. End-expiratory breath samples were collected at baseline (5 min prior to label administration) and every 30 min thereafter for 6 h[9]. For patients, a purpose-built connector on the ventilator tube was used to collect the breath samples directly into 10 mL glass tubes (Exetainer®, Buckinghamshire, UK). Healthy subjects were asked to exhale through a straw into the collection tubes. Breath samples were analyzed for 13CO2 concentration using an isotope ratio mass spectrometer (Europa Scientific, ABCA model 20/20, Crewe, UK). The values obtained were converted to percent dose recovery per hour, and used to calculate the area under the CO2 recovery curve (AUC 13CO2).
Manometric data obtained from patients were analyzed during five 4-h periods that corresponded to different stages within the 72 h post surgery: early ventilation (0-5 h), early post-operative (20-24 h), early feeding (29-33 h), late feeding (53-57 h), and late post-operative (68-72 h) (Table 1 and Figure 1). For healthy subjects, recordings were analyzed during fasting (d 1; 0-24 h) and enteral feeding (d 3; 48-72 h).
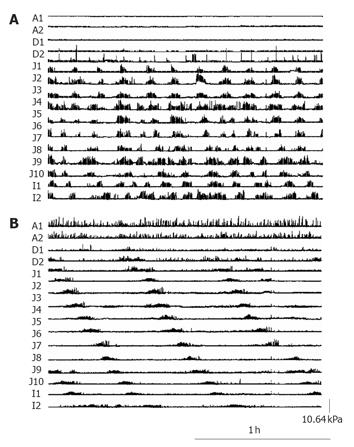
Motility recordings were analyzed manually to determine the frequency, site of origin (proximal/not proximal), distance of propagation and direction of travel of small intestinal burst activity. A burst was defined as the presence of ≥10 pressure waves per minute for at least 2 min, which migrated sequentially over four or more side-holes[11]. A threshold pressure wave amplitude >1.33 kPa measured from the end inspiratory pressure level to peak pressure was set to avoid confusion with respiratory pressure oscillations. Changes in pressure due to physical strains, which were recognized by having the same timing and amplitude in all channels, were excluded from the analysis. The spatial patterning of propagation of each burst sequence was classified as normal (antegrade, sequential) or abnormal (retrograde, bi-directional, simultaneous or multi-origin onset).
Analyses were performed using Statview Version 4.5 (Abacus Concepts Inc., CA, USA) software. The following parameters were analyzed: burst frequency (number of bursts per hour), percentage of normal bursts, percentage of bursts to start proximally, and the percentage of bursts to reach the ileum. Data were assessed using analysis of variance (AVOVA) to compare the data from different fasting periods. The Mann-Whitney U test was used to compare fasting and fed data, as well as patient and healthy data. Data for triolein absorption was compared using the Mann-Whitney U test. Data were expressed as mean±SE. A P < 0.05 was considered statistically significant.
The study protocol was well tolerated by all the subjects. Four patients reported minor local discomfort in the throat due to intubation of the manometry extrusion. The median APACHE II score[10] in patients was 11 (range 6-33). Major concomitant diseases were coronary artery disease (n = 7), hypertension (n = 2), chronic pulmonary disease (n = 3) and diabetes mellitus (n = 1). Manometric recordings were performed continuously for 72 h after surgery (range 66-121 h). Patients received mid-thoracic epidural anesthesia with 1.25 g/L marcain and l5 mg/L fentanyl for a median time of 66 h (range 21-75 h) after the operation.
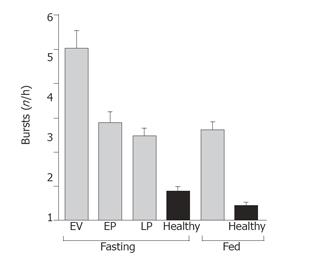
The frequency of small intestinal bursts was higher in patients under both fasting and feeding conditions, compared to healthy control subjects (Figure 2). During fasting, the frequency of bursts was six times greater in patients during the early ventilation period (0-5 h), compared to healthy controls (P < 0.0001, Figure 2). By day 3 (68-72 h), the burst frequency in patients had reduced, but was still three times greater than healthy subjects (P < 0.0001). Feeding suppressed the frequency of bursts by 23% in patients, compared to 50% in healthy subjects (Figure 2).
The percentage of bursts originating in the antrum was less in patients during fasting (P < 0.01) and enteral feeding (P < 0.05), compared to healthy control subjects (Table 2). Feeding had no effect on the number of bursts originating in the antrum in patients (70.0% ± 6.0% vs 65.0% ± 8.0%, early post-operative vs feeding) or healthy subjects (76.6% ± 14.3% vs 75.0% ± 11%, fasting vs feeding).
| Time period | % Bursts to startin the antrum | % Bursts propagatedto the ileum |
| Fasting | ||
| Patient: Early ventilation (1-5 h) | 70.0 ± 5.6b | 62.7 ± 9.3 |
| Early post-operative (20-24 h) | 70.1 ± 6.2b | 35.2 ± 8.6 |
| Late post-operative (68-72 h) | 75.5 ± 8.2b | 33.8 ± 8c |
| Healthy | 76.6 ± 14.3 | 43.7 ± 17.6 |
| Enteral feeding | ||
| Patient | 65.3 ± 7.6a | 53.9 ± 12.4 |
| Healthy | 75.0 ± 11 | 29.2 ± 12.0 |
A greater proportion of bursts reached the ileum in patients under both fasting and fed conditions, compared to healthy subjects (Table 2). In patients, the percentage of bursts reaching the ileum was higher during early ventilation (1-5 h) compared to the late post-operative (68-72 h) period (P < 0.05, Table 2).
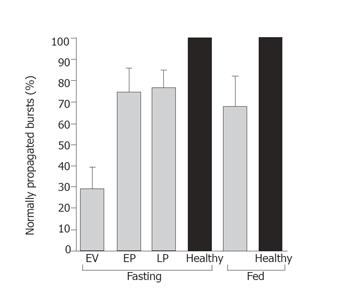
Bursts migrated normally in healthy subjects during both fasting and feeding conditions. In patients, the majority of bursts (71%) were abnormally propagated during the early ventilation period (1-5 h) (Figure 3); this proportion decreased over time so that by d 3 only 25% of bursts migrated abnormally (P < 0.01; early ventilation vs late post-operative) (Figures 3 and 4).
Absorption data were available for seven patients on d 1 post surgery, and four patients on d 3 due to minor tube displacement. Recovery of 13CO2 was slower in patients on d 1 post surgery than healthy control subjects (AUC 13CO2 1 323 ± 244 vs 2 646 ± 365; P < 0.05; respectively). In the four patients for whom data were available, 13CO2 recovery on day 3 post-surgery was one-fifth of the healthy controls (470 ± 832 vs 2 646 ± 365; P < 0.05; respectively). In patients, there was no difference in the recovery of 13CO2 between d 1 and 3 post surgery (Figure 5).
This is the first study to examine distal small intestinal motor activity in critically ill post-operative patients. The results show a transient disruption in the patterning of small intestinal motility extending as far as the ileum, in the immediate post-operative period. Ileal motor activity was present early after surgery, but was characterized by frequent bursts of abnormally propagated pressure waves, as seen in the proximal small intestine. These bursts of activity were not abolished by duodenal nutrient infusion. Previous studies in critically ill patients have demonstrated a disruption in small intestinal motor activity, with frequent and irregular bursts of activity in the duodenum and proximal jejunum[5,6]. The distance that these motor events migrated along the intestine was previously unknown. The recording sites in earlier studies were limited by lack of access to the distal intestine. In addition, the number of pressure sensors was restricted by the volume of perfusate necessary to undertake the recordings. The novel silicone rubber catheter extrusion used in the present study allowed very low rates of manometric perfusion so that recordings from the distal small bowel could be made from 16 sites simultaneously, with a total delivery of perfusate per 24 h of approximately 1 L. This is within the allowable tolerance for fluids in critically ill patients and minimizes the risk of fluid overload. Our data thus extends previous findings by demonstrating that the motor disruption in critically ill patients is generalized at least as far as the proximal ileum. In health, phase III activity has a maximum incidence in the proximal jejunum, with only a minority of MMCs reaching the ileum[12].
As previously reported, motor activity in critically ill patients is frequently abnormal[5,6]. In the present study, bursts of phase 3-like activity were seen extending to the ileum within the first 24 h post-surgery. The spatial organization of these bursts, however, were markedly abnormal, with both simultaneous onset and progression in a retrograde fashion, rather than a sequential migration down the gut that is typical of the fasting motor pattern. Over the subsequent 48 h, the organization of the bursts began to normalize and their frequency was reduced. However, the occurrence of bursts did not diminish during nutrient infusion, in contrast to the motor activity recorded from healthy subjects.
Enteral feeding remains as the preferred route of nutrition for critically ill patients[1]; however, effective delivery of feeds is often hampered by delayed gastric emptying[3,5]. Gastric dysmotility and stasis are likely to impair emptying both directly by the prevention of nutrient transit, and indirectly by the stimulation of feedback mechanisms that retard gastric motility. Some of these problems may be overcome by the use of post-pyloric feeding tubes; however, symptoms such as bloating, distension, and diarrhea remain common in patients receiving enteral nutrition. The effect of disordered small intestinal motility on the well-being of the patient is unclear.
There are limited data on the efficiency of the absorptive process in the critically ill. Singh et al[13] have previously demonstrated a severe depression in gut absorptive capacity for D-xylose in patients suffering from sepsis or trauma[13], particularly during the first 3-4 d of admission. Although the number of patients is limited in the present study, the results provide some insights into lipid absorption during critical illness. Our data suggest that lipid absorption may be impaired for at least 3 d following major surgery. The mechanisms responsible for these findings are unclear. It is likely that mucosal ischemia contributes to the disruption of the absorption process. However, the demonstration that small intestinal dysmotility extends to the ileum may also have consequences for absorption in these patients. The failure of conversion from a fasting to a fed motor pattern may disrupt the mixing of substrate with digestive enzymes that is normally seen with postprandial motility. We have previously shown a reduction in lipid but not glucose absorption when erythromycin is used to stimulate small intestinal burst activity in healthy human beings[8]. The motor patterns recorded at 72 h in the present study are likely to increase the rate of nutrient transit through the gut. Accelerated transit has been associated with reduced absorption in patients with ileostomies[14]. Changes in small intestinal motor function may therefore have important implications for the absorption of enteral nutrition in these patients. Further studies are necessary to examine the relative contributions of mucosal and motor dysfunction in the critically ill.
The mechanisms underlying the motor patterns recorded in the present study are unknown. Although all patients had manipulation of the bowel during the placement of the catheter, this is unlikely to explain the findings, as similar motor patterns have been reported in the proximal intestine of critically ill patients who have not undergone surgery[5,6]. It is also possible that drugs administered during routine clinical care may contribute to the observed motor dysfunctions. Opiates such as morphine are well recognized to produce burst activity. However, the majority of patients in our study received analgesia via an epidural catheter and required minimal or no systemic opiates during their ICU admission. Patients who required additional sedation for tolerance of mechanical ventilation usually received propofol. The effects of epidural anesthesia on motility are unclear. Although more rapid small intestinal transit has been reported when patients received epidural anesthesia instead of systemic opiates[15], spinal or epidural anesthesia had no effect on orocecal transit times in healthy volunteers[16]. It has been proposed that the fasting small intestine is under tonic inhibition by predominantly nitrergic mechanisms. It is thus possible that acute metabolic insults associated with critical illness disrupts these inhibitory pathways resulting in the release of motor activity, until control mechanisms recover.
In conclusion, we have shown that abnormal small intestinal motor activity in critically ill patients extends as far as the ileum. The disruption to the organization of fasting motor patterns has improved by d 3 post surgery, but the motor response to intestinal nutrients, and in particular, the conversion to postprandial motor activity remains impaired. The effects of these motor abnormalities on nutrient transit and absorption, together with their contribution to symptoms requires further study, as do the mechanisms underlying these dysfunctions.
S- Editor Guo SY and Pan BR L- Editor Elsevier HK E- Editor Bi L
| 1. | Heyland D, Cook DJ, Winder B, Brylowski L, Van deMark H, Guyatt G. Enteral nutrition in the critically ill patient: a prospective survey. Crit Care Med. 1995;23:1055-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 203] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 2. | Montejo JC. Enteral nutrition-related gastrointestinal complications in critically ill patients: a multicenter study. The Nutritional and Metabolic Working Group of the Spanish Society of Intensive Care Medicine and Coronary Units. Crit Care Med. 1999;27:1447-1453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 299] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 3. | Ritz MA, Fraser R, Edwards N, Di Matteo AC, Chapman M, Butler R, Cmielewski P, Tournadre JP, Davidson G, Dent J. Delayed gastric emptying in ventilated critically ill patients: measurement by 13 C-octanoic acid breath test. Crit Care Med. 2001;29:1744-1749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 89] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 4. | Montejo JC, Grau T, Acosta J, Ruiz-Santana S, Planas M, García-De-Lorenzo A, Mesejo A, Cervera M, Sánchez-Alvarez C, Núñez-Ruiz R. Multicenter, prospective, randomized, single-blind study comparing the efficacy and gastrointestinal complications of early jejunal feeding with early gastric feeding in critically ill patients. Crit Care Med. 2002;30:796-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 177] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 5. | Chapman M, Fraser R, Vozzo R, Bryant L, Tam W, Nguyen N, Zacharakis B, Butler R, Davidson G, Horowitz M. Antro-pyloro-duodenal motor responses to gastric and duodenal nutrient in critically ill patients. Gut. 2005;54:1384-1390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 80] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Dive A, Moulart M, Jonard P, Jamart J, Mahieu P. Gastroduodenal motility in mechanically ventilated critically ill patients: a manometric study. Crit Care Med. 1994;22:441-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 131] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 7. | Ritz MA, Fraser R, Tam W, Dent J. Impacts and patterns of disturbed gastrointestinal function in critically ill patients. Am J Gastroenterol. 2000;95:3044-3052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 89] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | Bryant LK, Fraser RJ, Vozzo R, Zacharakis B, Matthews GM, Butler R. Stimulation of small intestinal burst activity in the postprandial state differentially affects lipid and glucose absorption in healthy adult humans. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1028-G1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Watkins JB, Klein PD, Schoeller DA, Kirschner BS, Park R, Perman JA. Diagnosis and differentiation of fat malabsorption in children using 13C-labeled lipids: trioctanoin, triolein, and palmitic acid breath tests. Gastroenterology. 1982;82:911-917. [PubMed] |
| 10. | Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10902] [Cited by in RCA: 11141] [Article Influence: 278.5] [Reference Citation Analysis (0)] |
| 11. | Russo A, Fraser R, Adachi K, Horowitz M, Boeckxstaens G. Evidence that nitric oxide mechanisms regulate small intestinal motility in humans. Gut. 1999;44:72-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 65] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Kellow JE, Borody TJ, Phillips SF, Tucker RL, Haddad AC. Human interdigestive motility: variations in patterns from esophagus to colon. Gastroenterology. 1986;91:386-395. [PubMed] |
| 13. | Singh G, Harkema JM, Mayberry AJ, Chaudry IH. Severe depression of gut absorptive capacity in patients following trauma or sepsis. J Trauma. 1994;36:803-88; discussion 803-88;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 26] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Holgate AM, Read NW. Relationship between small bowel transit time and absorption of a solid meal. Influence of metoclopramide, magnesium sulfate, and lactulose. Dig Dis Sci. 1983;28:812-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 105] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Ahn H, Bronge A, Johansson K, Ygge H, Lindhagen J. Effect of continuous postoperative epidural analgesia on intestinal motility. Br J Surg. 1988;75:1176-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 74] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Thorén T, Wattwil M, Järnerot G, Tanghöj H. Epidural and spinal anesthesia do not influence gastric emptying and small intestinal transit in volunteers. Reg Anesth. 1989;14:35-42. [PubMed] |