INTRODUCTION
Nonsteroidal anti-inflammatory drugs (NSAIDs) such as aspirin are widely used as anti-inflammatory, analgesic agents[1,2]. However, gastrointestinal injury is a serious adverse effect of NSAIDs, and effective strategies to protect the gastrointestinal mucosa are required. Many previous studies have investigated the mechanisms for the development of NSAIDs-induced gastric mucosal lesions[3-5]. NSAIDs may cause gastric lesions by inhibiting cyclooxygenase (COX) and reducing prostaglandin (PG) production, but the exact pathogenic mechanism remains to be elucidated[6]. Several investigators have reported that intraperitoneal injection of anti-neutrophil serum or immunoneutralization of adhesion molecules on neutrophils and endothelial cells significantly attenuates gastric mucosal injury induced by NSAIDs[7,8]. Therefore, activation and infiltration of neutrophils into the stomach appear to contribute to the gastric mucosal lesions induced by NSAIDs.
Adenosine is a primordial signaling molecule that elicits numerous physiological responses in all mammalian tissues. The receptor-mediated effects of adenosine are mediated by four G protein-coupled receptors (A1, A2A, A2B, and A3)[9,10] which are variably expressed on immune cells depending on cell type and species. A2A receptors are found on bone marrow derived cells including neutrophils, monocytes, macrophages, lymphocytes, platelets, and mast cells[11]. Activation of A2A receptors on immune cells produces a series of responses that in general can be categorized as anti-inflammatory effects[12]. It has been reported that the activation of A2A receptors attenuates ischemia/reperfusion injury in the heart, lung, liver, and kidney by reducing neutrophil accumulation, superoxide generation, inhibition of endothelial adherence, and expression of the adhesion molecules[13-16]. Furthermore, activation of A2A receptors on human monocytes and mouse macrophages inhibits secretion of the pro-inflammatory cytokines, IL-12 and TNF-α[17,18]. We have reported that ATL-146e reduces gastric mucosal lesions induced by water-immersion stress[19]. On the basis of this evidence, we hypothesized that the activation of adenosine A2A receptors would reduce gastric mucosal injury induced by aspirin. In this study, we used ATL-146e, a new compound that has been shown to be more potent and selective than the older A2A agonist, CGS21680. In binding assays, ATL-146e was found to bind with more than 100 times higher affinity to recombinant human A2A receptors (Ki = 0.2 nmol/L) than to the other three adenosine receptor subtypes, A1, A3 and A2B[20]. We have reported that a single intraperitoneal injection of ATL-146e into the rats could inhibit TNF-α and IL-1βproduction, neutrophil accumulation in gastric injury induced by aspirin without affecting mucosal prostaglandin E2 (PGE2) concentration.
MATERIALS AND METHODS
Animals
Male Sprague-Dawley rats weighing 250-300 g were fed on a standard laboratory diet and water ad libitum, and kept in cages in a temperature and humidity controlled room with a 12-h dark-light cycle before and during the experiment. Prior to the administration of aspirin, animals were deprived of food for 24 h but had free access to water. This experimental protocol was approved by the Akita University Animal Care Committee.
Chemicals
4-{3-[6-Amino-9-(5-ethylcarbamoyl-3,4-dihydroxy-tetrahydro-furan-2-yl)-9H-purin-2-yl]-prop-2-ynyl}-cyclohexanecarboxylic acid methyl ester (ATL-146e) with its purity >99% was provided as a gift from Jayson Rieger, PhD Adenosine Therapeutics, Charlottesville, VA, USA[21]. ATL-146e was dissolved in a small volume of dimethylsulfoxide and then diluted >100-fold with physiological saline just before the injection.
Effect of ATL-146e on aspirin-induced gastric mucosal injury
Aspirin-induced gastric injury was produced by intragastric administration of aspirin (200 mg/kg) and HCl (0.15 mol/L, 8 mL/kg). ATL-146e (2.5 or 5 μg/kg, n = 5 each group) or vehicle was injected intraperitoneally 30 min prior to the administration of aspirin. The animals were killed by stunning and cervical dislocation 3 h after the administration of aspirin and the stomach was removed. Gastric mucosal lesions were measured by two independent observers who were blinded to the treatment. The ulcer index was calculated as the sum of the lengths of all lesions[22].
Effect of ATL-146e on myeloperoxidase in gastric mucosa
Gastric mucosal myeloperoxidase (MPO) concentration was assayed to quantify the degree of neutrophil infiltration. Three hundred milligrams of scraped mucosa was homogenized for 30 s with a polytron homogenizer (PT 1200, Kinematica AG, Littau, Switzerland) in 1.0 mL of ice-cold 5g/L hexadecyltrimethylammonium bromide in 50 mmol/L phosphate buffer (pH 6.0). Hexadecyltrimethylammonium bromide was used to negate the pseudoperoxidase activity of hemoglobin and to solubilize membrane-bound MPO. The homogenate was sonicated (U50 IKA Werke GmbH and Co. KG, Staufen, Germany) for 10 s, freeze-thawed thrice and centrifuged at 18 000 g for 20 min. The supernatant was taken for the determination of the enzyme activity utilizing an ELISA kit (Bioxytech, Oxis International, Inc., Portland, OR, USA). The change in absorbance at 405 nm was measured with a spectrophotometer (Microplate reader model 3550, Bio-Rad, Hercules, CA, USA). The concentration of MPO was expressed as nanogram per milligram protein measured using Bradford’s method[23].
Effect of ATL-146e on gastric of TNF-α and IL-1β
One hundred milligrams of scraped mucosa was homogenized for 30 s with a polytron homogenizer (PT 1200, Kinematica AG, Littau, Switzerland) in 1.0 mL of ice-cold potassium phosphate buffer (pH 7.4). Aliquots of homogenate supernatants in PBS were obtained by centrifugation at 10 000 g for 10 min. Total protein was measured by Bradford’s method. Concentration of TNF-α and IL-1β in the supernatant of mucosal homogenates was determined by ELISA (R&D Systems Inc., Minneapolis, MN, USA) according to the manufacturer’s instructions. After color development, optimal density was measured with a microplate reader. The concentration of TNF-α and IL-1β was expressed as pictogram per milligram protein.
Effect of ATL-146e on gastric secretion
Gastric juice was collected using the pylorus ligation method[24]. Briefly, rats were fasted for 24 h, placed in restraint cages, and injected intraperitoneally with 5 μg/kg of ATL-146e. Thirty minutes after the injection, pylorus ligation was performed. The stomach of the rats were removed 3 h after pylorus ligation, and the gastric contents were collected. Following centrifugation, acid content in the supernatant determined by titration with 0.01 mol NaOH to pH 7.0 using a pH meter (Φ50 pH Meter; Beckman, Tokyo, Japan), was used to calculate gastric acid secretion in mmol/3 h.
Effect of ATL-146e on mucosal PGE2
To evaluate the effect of ATL-146e on mucosal content of PGE2, rats were divided into four groups. In group A, rats were treated with vehicle. In group B, rats were treated with 5 μg/kg of ATL-146e. Thirty minutes later, the animals were killed. In group C, vehicle was injected intraperitoneally 30 min prior to the administration of aspirin and were killed 3 h later. In group D, ATL-146e (5 μg/kg) was injected intraperitoneally 30 min prior to the administration of aspirin and killed 3 h later. A part of fundic mucosa (about 100 mg) was excised for the determination of PGE2 synthesis. The samples were weighed, finely minced with scissors for 15 s, and then suspended in 1.0 mL of 10 mmol/L sodium phosphate buffer (pH 7.4). The samples were then incubated in a shaking bath (37 °C) for 29 min followed by centrifugation at 9 000 g for 30 s. The supernatant was frozen and subsequent determination of PGE2 was performed by radioimmunoassay using PGE2 [125I] RIA kit (Dupont/NEN, Boston, MA, USA).
Statistical analysis
All data were expressed as mean±SE. Statistical significance was determined by ANOVA using Statview-J 4.11 statistical program (Abacus Concepts, Berkeley, CA, USA). P < 0.05 was considered statistically significant.
RESULTS
Effect of ATL-146e on aspirin-induced gastric lesions
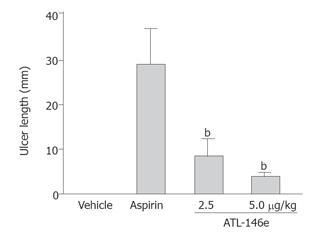
Administration of aspirin resulted in the appearance of linear and dotted erosions in the gastric mucosa of vehicle-treated rats. In contrast, pre-treatment with ATL-146e resulted in smaller erosions. The total length of gastric erosions (ulcer index) in control rats was 29.8 ± 7.7 mm. The ulcer index in rats pretreated with ATL-146e was significantly suppressed to 7.0 ± 2.34 mm (2.5 μg/kg, P < 0.05), or 3.1 ± 1.42 mm (5.0 μg/kg, P < 0.01) (Figure 1).
Figure 1 Ulcer length of rats 3 h after the administration of aspirin with or without pretreatment with ATL-146e (2.
5 or 5 μg/kg, ip) (n = 5 each group). bP < 0.01 vs aspirin alone treated group.
Effect of ATL-146e on MPO in gastric mucosa
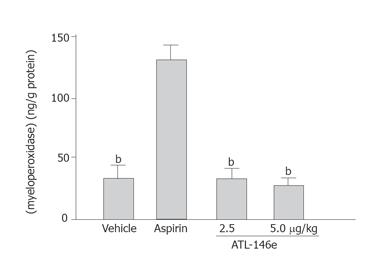
Tissue MPO concentration in the gastric mucosa increased 3 h after the initiation of administration of aspirin. The MPO concentration in normal control animals (2.8 ± 0.2 μg/g protein) increased to 13.8 ± 1.2 μg/g protein in vehicle-treated rats. The increment of MPO concentration in the gastric mucosa by aspirin was suppressed by pretreatment with ATL-146e to 2.9 ± 0.35 μg/g protein (2.5 μg/kg, P < 0.001) or 2.7 ± 0.14 μg/g protein (5 μg/kg, P < 0.001) compared to that in vehicle-treated rats (Figure 2). The increase in MPO above control levels caused by aspirin was reduced to nearly normal levels after the administration of 2.5 and 5 μg/kg ATL-146e.
Figure 2 Effect of ATL-146e on myeloperoxidase concentration in gastric mucosa (n = 5 each group).
bP < 0.001 vs aspirin alone treated group.
Effect of ATL-146e on gastric of TNF-α and IL-1β
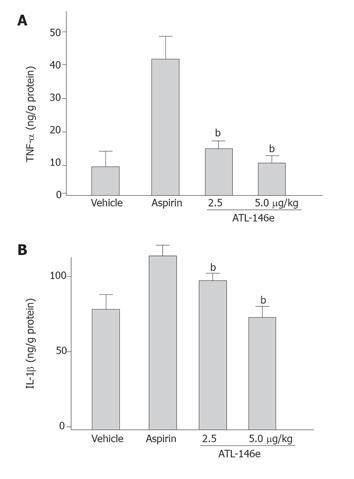
The gastric concentrations of TNF-α and IL-1β were significantly increased 3 h after the administration of aspirin. ATL-146e at the doses of 2.5 and 5.0 μg/kg significantly suppressed the increment of tissue TNF-α and IL-1β in the gastric mucosa by the administration of aspirin (Figure 3). The increase in TNF-α above the control levels caused by aspirin was reduced by the administration of 2.5 and 5 μg/kg ATL-146e by 90.3% and 99.7%, respectively. The increase in IL1-β above the control levels caused by aspirin was reduced by the administration of 2.5 and 5 μg/kg ATL-146e by 64.0% and 97.2%, respectively.
Figure 3 Effect of ATL-146e on increased TNF-α (A) and IL-1β (B) concentration in gastric mucosa induced by aspirin administration (n = 5 each group).
bP < 0.01, vs aspirin alone treated group.
Effect of ATL-146e on gastric secretion
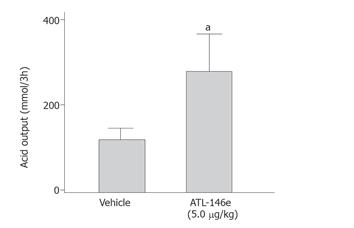
ATL-146e caused a three-fold increase in gastric acid output from 114 to 326 mmol/3h (P < 0.05, Figure 4).
Figure 4 Effect of 5 μg/kg ATL-146e on gastric acid secretion.
Rats were treated with or without 5.0 μg/kg of ATL-146e and killed 3 h later (n = 5 each group). aP < 0.05 vs vehicle treated group.
Effect of ATL-146e on mucosal of PGE2
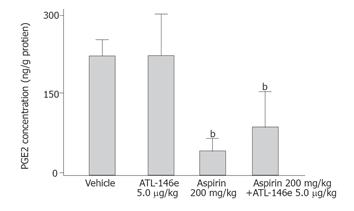
The concentration of PGE2 was 255.3 ± 64.7 ng/g in vehicle-treated rats (group A) and 47.4 ± 5.6 ng/g in aspirin-treated animals (group C) (82.3% reduction, P < 0.05). ATL-146e (5 μg/kg) administration did not interfere with the reduction of gastric PGE2 concentration induced by aspirin (P > 0.05 vs aspirin alone) (Figure 5).
Figure 5 Gastric mucosal concentrations of prostaglandin E2 in control group (vehicle group) and in rats treated with 200 mg/kg aspirin alone or pretreated with 5 μg/kg ATL-146e (n = 5 each group).
bP < 0.01 vs vehicle treated group. NS: no significant difference between each group.
DISCUSSION
The results of this study clearly showed that a single bolus injection of the adenosine A2A agonist, ATL-146e 30 min prior to the administration of aspirin, could effectively reduce the extent of gastric mucosal lesions. This protection is correlated with the inhibition of neutrophil infiltration into the gastric mucosal tissue and production of pro-inflammatory cytokines in gastric mucosal tissue. Previous studies have shown that gastric mucosal MPO concentration, a biochemical indicator of neutrophils, increases with the development of gastric mucosal lesions[25]. These findings suggest that most of the stimuli for aspirin-induced gastric injury that is susceptible to the inhibition by ATL-146e may occur early after the administration of aspirin.
Recent studies reported that gastric mucosal lesions can be reduced by the administration of antibodies against TNF-α[26]. Therefore, we may postulate that infiltration of neutrophils in rat gastric mucosa after the administration of aspirin could occur in response to the gastric production of pro-inflammatory cytokines, resulting in the development of gastric mucosal lesions secondary to neutrophil accumulation. Anti-inflammatory and tissue protective effects of ATL-146e in the models of ischemia-reperfusion injury have been reported[13,14,27-29].
Adenosine has been identified as an endogenous anti-inflammatory agent because the activation of the A2A receptor is known to increase intracellular cAMP levels and to reduce diverse leukocyte functions[30]. Ross et al[14] demonstrated that ATL-146e protects lung from reperfusion injury by reducing neutrophil sequestration[14]. Our recent study reported that ATL-146e inhibits water-immersion stress-induced gastric injury due to the inhibition of neutrophil accumulation and reduction of pro-inflammatory cytokine production[19]. These findings led us to examine the effect of ATL-146e on aspirin-induced gastric mucosal lesion. In the present study, we have demonstrated that MPO concentration, an index of tissue-associated neutrophil accumulation, increased in the gastric mucosa 3 h after the administration of aspirin. The increased MPO concentration was significantly inhibited by treatment with ATL-146e.
TNF-α is a pro-inflammatory cytokine and has recently been shown to be a crucial mediator of NSAIDs-induced gastric mucosal injury[31]. Also, TNF-α is a cytokine that strongly stimulates neutrophil adherence by inducing synthesis and expression of adhesion molecules on endothelial cells and neutrophils[32,33]. TNF-α augments neutrophil-derived superoxide generation and upregulates the expression of adhesion molecules on neutrophil and endothelium, and stimulates production of IL-1β, leading to neutrophil accumulation[32,33]. Furthermore, studies on experimental models have shown that intravenous administration of TNF-α produces extensive neutrophil infiltration within the microvasculature of the digestive tract. In addition, portal infusion of TNF-α causes gastric and small intestinal damage in rats[34]. The present study demonstrated that ATL-146e treatment could inhibit increase of TNF-α and IL-1β concentration in the gastric mucosa after the administration of aspirin. These findings strongly support the hypothesis that ATL-146e attenuates aspirin-induced neutrophil accumulation by inhibiting production of pro-inflammatory cytokines.
Okusa et al[13] reported that ATL-146e inhibits ischemic reperfusion injury of kidney not only by reducing neutrophil accumulation, but also by reducing the expression of the adhesion molecules, P-selectin, and ICAM-1 on the reperfused vascular endothelium. Andrews et al[6] also reported that the expression of ICAM-1 on endothelial cells is increased by NSAIDs. Therefore, the anti-ulcer effect of ATL-146e might be due to the inhibition of neutrophil adhesion.
In addition, it is well established that various anti-secretory agents, such as H2-receptor antagonists and proton pump inhibitors prevent gastric lesions[35]. However, we found that ATL-146e could significantly increase gastric acid secretion. These data indicate that the protective effect of ATL-146e is not due to reduced gastric acid secretion. Also, it has been reported that PGE2 prevents gastric mucosal damage by aspirin in human beings and animals[36]. The protective effect of ATL-146e is not dependent on gastric mucosal prostaglandin synthesis, since pretreatment with ATL-146e, which reduces gastric damage, has no effect on the gastric mucosal prostaglandin concentration. Therefore, inhibition of mucosal lesions by ATL-146e cannot be attributed to gastric acid inhibition and prostaglandin synthesis.
In conclusion, the potent and selective adenosine A2A receptor agonist, ATL-146e, significantly inhibits acute gastric mucosal injury induced by aspirin in rats. This effect may be due in part to a reduction in neutrophil infiltration into the gastric mucosa and inhibition of pro-inflammatory cytokines production. Also, modulation of adenosine A2A receptor activity by specific agonists may be clinically useful for the therapy of NSAIDs-induced gastric mucosal damage.