Published online Jan 28, 2006. doi: 10.3748/wjg.v12.i4.561
Revised: July 8, 2005
Accepted: July 20, 2005
Published online: January 28, 2006
AIM: To determine the efficacy of long-term lamivudine treatment of a large number of Japanese patients with chronic hepatitis B.
METHODS: In this retrospective, multi-center trial, 318 Japanese patients with chronic hepatitis B received 100 mg of lamivudine daily for up to 36 (median 21) mo. Virological response was a decline to a serum HBV DNA level less than 3.7 log copies/mL. Virological breakthrough was defined as the reappearance of a serum HBV DNA level to more than 10-fold the minimum during treatment.
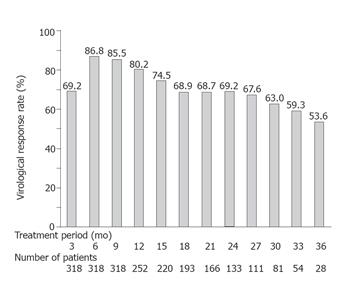
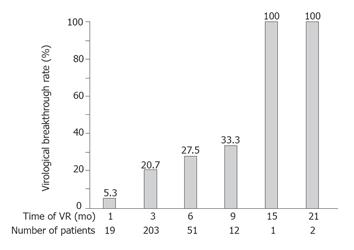
RESULTS: Lamivudine produced virological response in 86.8% of the 318 patients at 6 mo, in 80.2% of 252 patients at 12 mo, in 69.2% of 133 patients at 24 mo, and in 53.6% of 28 patients at 36 mo. Forward stepwise logistic regression analysis showed an HBV DNA level less than 6.8 log copies/mL (P < 0.0001), HBeAg negativity (P < 0.0001), a platelet count of 100 × 109/L or more (P = 0.0162) at baseline, and a decline of the HBV DNA level of more than 3.2 log copies/mL as compared with the baseline level at 3 mo after the start of treatment (P = 0.0003) to be significantly associated with virological response. Among patients with a virological response, virological breakthrough was seen in 5.3% of 19 patients who responded virologically at 1 mo, in 20.7% of 203 patients at 3 mo, in 27.5% of 51 patients at 6 mo, in 33.3% of 12 patients at 9 mo, and in 100% of 3 patients at ≥15 mo. A virological breakthrough was found significantly more often in patients with delayed virological response.
CONCLUSION: Lamivudine treatment could suppress serum HBV DNA in most of the tested Japanese patients. Long-term efficacy might be seen in patients without HBeAg at baseline, in the absence of cirrhosis, and in patients with a decline in HBV DNA level soon after the start of treatment.
- Citation: Furusyo N, Takeoka H, Toyoda K, Murata M, Tanabe Y, Kajiwara E, Shimono J, Masumoto A, Maruyama T, Nomura H, Nakamuta M, Takahashi K, Shimoda S, Azuma K, Sakai H, Hayashi J, Group TKULDS. Long-term lamivudine treatment for chronic hepatitis B in Japanese patients: A project of Kyushu University Liver Disease Study. World J Gastroenterol 2006; 12(4): 561-567
- URL: https://www.wjgnet.com/1007-9327/full/v12/i4/561.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i4.561
Chronic hepatitis B virus (HBV) infection affects 400 million people worldwide, three-quarters of whom reside in Asia[1]. The morbidity and mortality of chronic HBV infection are a major public health concern. Vertical transmission of HBV is the main cause of chronic HBV infection in the endemic areas of Asia. In Japan which is endemic for HBV infection, transmission can be easily prevented by vaccination of at risk-infants. The prevalence of hepatitis B surface antigen (HBsAg) carriage in the general population was reported to be less than 2%[2]. However, in areas of high HBV endemicity, persons with HBV-related cirrhosis have an approximately threefold higher risk of hepatocellular carcinoma (HCC) than those with chronic hepatitis but without cirrhosis and a 16-fold higher risk of HCC than the carriers in whom the virus is inactive[3]. Interferon-alpha (IFN-α) has been approved for the treatment of chronic hepatitis B[4], but it is poorly tolerated and effective in only 20%-30% of patients. Since the late 1990s, several studies have demonstrated the effectiveness of the initially available antiviral medicine, lamivudine, for patients with chronic hepatitis B: HBV DNA suppression, normalization of alanine aminotransferase (ALT), loss of hepatitis B e antigen (HBeAg). Moreover, studies have demonstrated the improvement of hepatic histology by the administration of lamivudine compared to placebo[5-8].
Lamivudine, an oral cytosine nucleoside analog, is the (-) - beta-enantiomer of 2’,3’-dideoxy-3’-thiacytidine. HBV replicates through a pregenomic RNA intermediate. Lamivudine interferes with HBV reverse transcriptase (DNA polymerase) activity and causes chain termination of nascent viral DNA, leading to the inhibition of HBV replication[9]. Long-term treatment with lamivudine is not an option because it leads to drug resistance in most cases[10,11]. Lamivudine treatment, especially for chronic HBV-infected patients with cirrhosis, may also act as a bridge to more definitive treatments, such as liver transplantation. However, in several countries, including Japan, liver transplantation is not easily available because of insufficiency of donors, and even in other countries, many patients have to wait for long periods for transplantation. Although several non-Asian studies, from North America and Europe, have shown the efficacy of long-term use of lamivudine[12,13], few studies have assessed the efficacy of long-term lamivudine treatment of a large number of Japanese patients with chronic hepatitis B.
To acquire more data on these issues, 37 Japanese liver units involved in the management of HBV-related chronic liver diseases in Kyushu, Japan cooperated in this study. The objective of the present study was to analyze the results of long-term lamivudine administration for the suppression of HBV replication and the clinical outcomes of a large number of Japanese patients with chronic hepatitis B.
This retrospective analysis encompassed 318 Japanese chronic hepatitis B patients (231 males and 87 females, mean age 47.8 years) on lamivudine monotherapy for up to 36 (median 21, range 9-36) mo. Clinical features from 403 HBsAg-positive patients with chronic liver diseases, who started lamivudine treatment between December 2000 and March 2004 in 37 Japanese liver units in Kyushu, were recorded in a centralized database. All patients were determined to be serum HBV DNA-positive via polymerase chain reaction (PCR) assay prior to treatment. The diagnosis of chronic hepatitis and cirrhosis was based on a liver biopsy in most patients, if unavailable, on clinical laboratory, and ultrasound data. Eighty five patients were excluded from the present analysis because of one or more of the following reasons: age below 18 years; positive for antibody to hepatitis C virus or human immunodeficiency virus type 1; diagnosis of HCC within 3 mo after enrolment; time of lamivudine treatment within 9 mo; or treatment with anticancer drugs or corticosteroid drugs for other malignancies, such as leukemia, lymphoma or autoimmune diseases. Because this was a retrospective analysis of treated patients, there were no predefined criteria for treatment withdrawal or combination treatments. Criteria for withdrawal and combination treatments after the start of the treatment were dependent upon the strategy used by the physician at each center. In the present study, follow-up was stopped for patients who discontinued lamivudine treatment or started receiving a combination treatment with IFN and lamivudine, or with adefovir dipivoxil and lamivudine.
The patients received lamivudine (Zeffix®, Glaxo Smith Kline, UK) orally in a single daily dose of 100 mg. Data concerning age, sex, history of prior IFN treatment, Child-Turcotte-Pugh (CTP) score, series of serum laboratory testing of ALT, total bilirubin, albumin, HBeAg, and HBV DNA level were collected. Also, we analyzed virological (time of virological response and virological breakthrough) and biological events (time of ALT normalization, ALT breakthrough, and hepatitis flare) during the observation period. The clinical events recorded were hepatic decompensation (ascites, portal hypertensive bleeding, and hepatic encephalopathy) and liver-related death during the study period.
Quantification of serum HBV DNA was performed at each center using one of the following commercial assays according to local availability: quantitative PCR assay (Amplicor HBV Monitor, Roche Diagnostics, Mannheim, Germany), over a detection range from 2.6 (corresponding to 400 copies/mL) to 7.5 log copies/mL; or transcription-mediated amplification and hybridization protect assay (TMA-HPA, Chugai Diagnostics, Tokyo, Japan), over a detection range from 3.7 log genome equivalents (LGE)/mL (corresponding to 5 000 copies/mL) to 8.7 LGE/mL. A decline of the serum HBV DNA level to less than 3.7 log copies/mL during treatment was considered as a virological response. Virological breakthrough was defined as the reappearance of a serum HBV DNA level to more than 10-fold the minimum during treatment. We analyzed whether or not an early decline of the HBV DNA level at 3 and 6 mo after the start of the treatment was related to virological response and breakthrough.
The serum ALT, bilirubin, and albumin levels were serially determined using the standard method every month before treatment and during the treatment. The upper normal limits for the ALT level were slightly different in each facility, ranging between 30 and 40 IU/mL. Normalization with an ALT level 667 or below during the treatment was considered as a biological response. A deterioration of ALT to an abnormal level after normalization during the treatment was considered as an ALT breakthrough. A deterioration of the ALT level more than 10 times the upper limit of normal (ULN) was considered as a hepatitis flare.
Categorical variables were analyzed using χ2 test or Fisher’s exact test. The Mann-Whitney U-test was also used to compare responders and non-responders with regard to various characteristics, when appropriate. The Cochran-Armitage’s trend test was used to determine the relationship between the increases or decreases in the virological breakthrough rates of patients with virological response. Independent factors associated with responders were studied using forward stepwise logistic regression analysis of the following variables: age at the start of treatment, sex, history of prior IFN treatment, histological staging and grading, pretreatment laboratory data, serum pretreatment HBV DNA level, and the median declines of HBV DNA level at 3 and 6 mo after the start of the treatment. Forward stepwise logistic regression analysis was performed using a commercially available software package (BMDP Statistical Software Inc., Los Angeles, CA, USA) for the IBM 3090 system computer. The BMDP program LR was used to evaluate the relationship between the clinical features and SVR. Using this method, the most significant associated variable was entered into the model. After adjusting for that variable, the next most significant variable was added to the model. Two-tailed P values less than 0.05 were considered statistically significant.
The mean age and percentage over 35 years were significantly higher in patients with cirrhosis than in those without cirrhosis, while the mean ALT level, albumin, and platelet counts were significantly higher in patients without cirrhosis than in those with cirrhosis. No significant differences in sex distribution, total bilirubin, positivity of HBeAg, or HBV DNA level were observed between these groups (Table 1). This study consisted of 173 HBeAg-positive and 145 HBeAg-negative patients with a mean pretreatment HBV DNA level of 6.8±1.2 (median 7.0) log copies/mL. Concerning the relationship between HBeAg and HBV DNA level, the mean HBV DNA level was significantly higher in HBeAg-positive patients with (7.3 ± 1.1 log copies/mL) and without cirrhosis (7.2 ± 1.0 log copies/mL) as compared with HBeAg-negative patients with (6.3±1.2 log copies/mL) and without cirrhosis (6.2 ± 1.1 log copies/mL) (both P < 0.0001). No significant difference in HBV DNA level, even classified by HBeAg status, was observed between patients with and without cirrhosis.
| Characteristics | Cirrhosis | P | |
| No | Yes | ||
| n = 216 | n = 102 | ||
| Number of men (%) | 154 (71.3) | 77 (75.5) | 0.5168 |
| Age (yr) | 45.0 ± 11.1 | 53.7 ± 9.7 | < 0.0001 |
| Number with 35 yr old and over (%) | 173 (80.1) | 98 (96.1) | 0.0003 |
| ALT (IU/L) | 320.9 ± 503.3 | 101.5 ± 95.4 | < 0.0001 |
| Total bilirubin (mg/dL) | 1.2 ± 1.3 | 1.4 ± 1.3 | 0.188 |
| Albumin (g/dL) | 4.0 ± 0.4 | 3.5 ± 0.6 | < 0.0001 |
| Platelet count (mean ± SD) (x104/μL) | 16.2 ± 5.3 | 9.8 ± 4.4 | < 0.0001 |
| Number of HBeAg positivity (%) | 119 (55.1) | 54 (52.9) | 0.8112 |
| HBV-DNA1 | 6.8 ± 1.3 | 6.6 ± 1.2 | 0.1344 |
| Lamivudine treatment (mo) | 20.2 ± 8.9 | 21.8 ± 9.7 | 0.1429 |
In analyses of an early decline of HBV DNA level by lamivudine, the mean declines of all the studied patients were 3.2 ± 1.2 (median 3.2) log copies/mL at 3 mo and 3.6 ± 1.1 (median 3.8)log copies/mL at 6 mo after the start of treatment. During the treatment period of up to 36 (median 21) mo, a virological response was found in 90.6% (288/318) patients and ALT normalization was found in 86.2% (274/318) patients. Of the 288 with virological response, 255 (88.5%) had ALT normalization. Of the remaining 30 without virological response, 19 (63.3%), who had achieved virological suppression with a low HBV DNA level of 3.7-4.0 log copies/mL by lamivudine, had ALT normalization, but 11 (36.7%) had no ALT normalization and an HBV DNA level of more than 4.0 log copies/mL.
The mean pretreatment HBV DNA level was significantly lower in patients with virological response (6.6 ± 1.2 log copies/mL) than those without virological response (7.7 ± 0.7 log copies/mL) (P < 0.0001). The frequency of pretreatment HBeAg positivity was significantly lower in patients with virological response (51.0%, 147/288) than those without virological response (86.7%, 26/30) (P = 0.0004). No significant differences in sex distribution, age, ALT level, platelet count, presence of cirrhosis, or CTP score were found between the patients with and without virological response (Table 2).
| Characteristics | Virological response | P | |
| No | Yes | ||
| n = 288 | n = 30 | ||
| Number of men (%) | 207 (71.9) | 24 (80.0) | 0.4624 |
| Age (yr) | 47.9 ± 11.4 | 46.9 (22-73) | 0.6453 |
| Number of cirrhosis (%) | 90 (31.2) | 12 (40.0) | 0.4403 |
| Baseline laboratory data | |||
| Total bilirubin (mg/dL) | 1.3 ± 1.5 | 1.1 ± 0.6 | 0.6895 |
| Albumin (g/dL) | 3.9 ± 0.6 | 3.8 ± 0.6 | 0.2381 |
| Platelet count (x 104/μL) | 14.3 ± 5.9 | 13.2 ± 5.8 | 0.2624 |
| Number of HBeAg positivity (%) | 147 (51.0) | 26 (86.7) | 0.0004 |
| HBV-DNA1 | 6.6 ± 1.2 | 7.7 ± 0.7 | < 0.0001 |
Lamivudine suppressed serum HBV DNA to less than 3.7 log copies/mL in 69.2% patients at 3 mo, in 86.8% patients at 6 mo, in 80.2% patients at 12 mo, in 69.2% patients at 24 mo, and in 53.6% patients at 36 mo. The efficacy rate of virological response decreased with the length of the treatment period of patients who received lamivudine for over 6 mo (Figure 1).
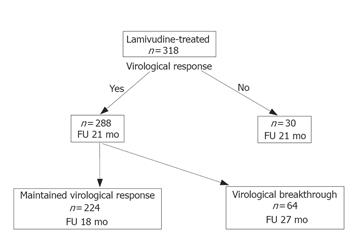
Of the 288 patients with virological response, 224 (77.8%) had a sustained virological response, and 64 (22.2%) had a virological breakthrough. The median follow-up time was significantly shorter for patients with sustained virological response (18 mo) than those with virological breakthrough (27 mo). The frequencies of pretreatment HBeAg positivity [65.6% (42/64) vs 46.8% (105/224); P = 0.0123] and cirrhosis [43.8% (28/64) vs 27.7% (62/224); P = 0.0218] were significantly higher for patients with virological breakthrough than those without a breakthrough (Figure 2). No significant differences in sex distribution, age, or pretreatment HBV DNA level were observed between these groups.
Of the 318 patients, 173 (54.4%) were detected to have HBeAg in their sera at baseline. Of the 173 HBeAg-positive patients, 82 (47.4%) had clearance of HBeAg and 91 (52.6%) continued to have HBeAg during treatment. Lamivudine led to HBeAg clearance by 6.9% of the patients at 1 mo, by 24.9% of the patients at 3 mo, by 32.9% at 6 mo, by 35.8% at 9 mo, by 37.6% at 12 mo, by 45.1% at 18 mo, and by 47.4% at 36 mo, suggesting that HBeAg clearance rates increased with the duration of lamivudine treatment (Figure 3). HBeAg clearance always occurred after virological response in all the 82 who cleared HBeAg. No significant differences in sex distribution, age, ALT level, platelet count, presence of cirrhosis, or CTP score were found between the patients with and without HBeAg clearance. Of the 145 patients with HBeAg negative at baseline, no patient reversed to HBeAg positive. We observed that the patients who cleared HBeAg (79/82, 96.3%) and the patients with HBeAg negative at baseline (141/145, 97.2%) had a significantly higher virological response rate than those without HBeAg clearance (68/91, 74.8%, P = 0.0002, P < 0.0001, respectively).
Of the 274 patients with ALT normalization by lamivudine, 231 (84.3%) had sustained ALT normalization, and 43 (15.7%) had an ALT breakthrough. Of the 43 patients with an ALT breakthrough, 4 (9.3%) had a hepatitis flare: 4 males, 3 with cirrhosis and 1 without cirrhosis, and 4 with HBeAg. However, no patient with hepatic decompensation, who had marked hyperbilirubinemia, or had a liver-related death, was observed in this study. The time of ALT changes always depended on the time of virological change: ALT deterioration after normalization followed an increase in the HBV DNA level in all cases. The frequency of HBeAg positivity at baseline was significantly higher in patients with a breakthrough than those without a breakthrough [72.1% (37/43) vs 51.5% (119/231); P < 0.0001]. No significant differences in sex distribution, age, pretreatment HBV DNA level, presence of cirrhosis or CTP score were observed between these groups.
Among the 288 with virological response, virological breakthrough was seen in 1 (5.3%) of 19 who had virological response at one month, in 42 (20.7%) of 203 at 3 mo, in 14 (27.5%) of 51 at 6 mo, in 4 (33.3%) of 12 at 9 mo, and in 3 (100%) of 3 at ≥15 mo. Cochran-Armitage’s trend test revealed that virological breakthrough was significantly more prevalent in patients with delayed virological response (P < 0.0001) (Figure 4).
At baseline, an HBV DNA level less than 6.8 log copies/mL (P < 0.0001), HBeAg negativity (P < 0.0001), and platelets count of 100×109/L or more (P = 0.0224) were significantly associated with virological response in the 318 studied patients (Table 3). Of the treatment factors, an early decline of 3.2 or more log copies/mL of HBV DNA at 3 mo after the start of the treatment was significantly associated with the response (P < 0.0001).
| Factors | Odds ratio | 95% CI | P |
| (At baseline) | |||
| HBV DNA less than 6.81 | 434.7 | 104.1 - 2000 | < 0.0001 |
| HBeAg negativity | 7.142 | 2.136 - 238.0 | < 0.0001 |
| Platelet count more than 10 0x 109/L | 4.625 | 1.242 - 17.22 | 0.0224 |
| (During treatment) | |||
| Decline of HBV-DNA more than 3.21 | 51.13 | 11.21 - 233.0 | < 0.0001 |
| within 3 mo of the start of treatment |
At baseline, cirrhosis (P = 0.008), HBeAg positivity (P = 0.0085), and platelets count less than 100 × 109/L (P = 0.0491) were significantly associated with a virological breakthrough in the 288 patients with virological response (Table 4). Of the treatment factors, an early decline of 3.8 or less log copies/mL of HBV DNA at 6 mo after the start of the treatment was significantly associated with the breakthrough (P = 0.0084).
| Factors | Odds ratio | 95% CI | P |
| (At baseline) | |||
| Cirrhosis | 3.527 | 1.687 - 7.371 | 0.0008 |
| HBeAg positivity | 2.512 | 1.265 - 4.989 | 0.0085 |
| Platelet counts less than 100 x 109/L | 2.386 | 1.003 - 5.676 | 0.0491 |
| (During treatment) | |||
| Decline of HBV-DNA less than 3.91 | 2.358 | 1.246 - 4.464 | 0.0084 |
| within 6 mo of the start of treatment |
To our best knowledge, no such large-scale studies as this of lamivudine have been carried out for Japanese chronic hepatitis B patients. In this retrospective study, good virological and biological efficacy for up to 36 mo of lamivudine treatment was seen in Japanese patients with chronic hepatitis B, with no relation to sex, age, or ALT level at baseline. The effect was sustained for the patients with HBeAg-negative before treatment, absence of cirrhosis, and with an early decline of the HBV DNA level after the start of the treatment. During the treatment, very few patients with a hepatitis flare were seen and none with hepatic decompensation, marked hyperbilirubinemia, or liver-related death were seen in this study. The aims of treatment for chronic hepatitis B are to achieve sustained suppression of HBV replication and remission of inflammation in the liver. The antiviral responses for chronic hepatitis B are categorized as biochemical (ALT normalization), virological (decrease of HBV DNA to less than 5 log copies/mL and loss of HBeAg), and histological, and as on-therapy or sustained off-therapy[14]. Treatment for chronic hepatitis B patients seems to be necessary when the HBV DNA level exceeds 5 log copies/mL, independent of ALT activity[11]. Lamivudine well inhibited HBV DNA replication in Japanese chronic hepatitis B patients.
HBeAg clearance usually predicts long-lasting suppression of HBV, reduced infectivity and an improved clinical prognosis[15]. In this study, 47.4% of patients with HBeAg at baseline had HBeAg eliminated from their sera. Follow-up reports of the multicenter Asian study for Chinese patients showed that HBeAg clearance rates increased with the duration of lamivudine treatment, from 17% to 22% at 12 mo, 27 to 29% at 24 mo, and 33 to 40% at 36 mo[12,16,17]. The results of our study were consistent with those of these non-Japanese patient, although the HBeAg clearance rates within 24 mo were relatively high in our study. Lamivudine was effective in terms of HBeAg clearance in Japanese chronic hepatitis B patients. Patients successfully treated for chronic hepatitis B are less likely to develop cirrhosis, liver failure, and HCC in comparison with those who do not respond to treatment[18]. A randomized controlled trial of lamivudine for chronic hepatitis B patients demonstrated that HCC incidence was reduced by lamivudine antiviral therapy, showing an incidence of 3.9% in lamivudine-treated patients and 7.4% in a placebo control group, with a hazard ratio of 0.49 (95%CI = 0.25-0.99)[19]. For chronic hepatitis B patients, antiviral therapy with lamivudine that results in sustained suppression of HBV DNA replication and hepatic necroinflammation may reduce the incidence of HCC.
It has been reported that resistance to lamivudine often develops after 6 mo of treatment[10]. The present study was limited in its value because we detected viruses resistant to lamivudine. However, in our study, the emergence of resistant viruses could be defined by the virological breakthrough (reappearance of serum HBV DNA levels more than 10-fold increase from the minimum). A serious drawback of long-term lamivudine treatment is the development of resistant HBV mutants, i.e., the mutations in a tyrosine-methionine-aspartate-aspartate (YMDD) motif of the HBV polymerase gene, associated with increase in serum HBV DNA and the ALT level[19]. The present study showed that the HBV DNA suppression rates by lamivudine decreased with the duration of treatment, but that a relapse of biochemical response, ALT breakthrough was found only in 15.7% of patients during these treatment periods. Lamivudine treatment withdrawal can cause HBV DNA to revert to pretreatment levels, with the relapse of clinical hepatitis[20]. With the excellent safety and tolerability of lamivudine, continuous therapy is suggested as beneficial[4]. After the start of phylogenetic analyses, based on inter group divergence of 8% or more over the complete HBV nucleotide sequence, seven different genotypes, arbitrarily designed A-G, have been recognized[21,22]. Several reports have shown geographical distribution of the genotypes, with genotypes A and D predominant in Western Europe, B and C in South Asia and the Far East, and F in South America[21-26]. Due to the geographical distribution pattern, HBV genotypes B and C are commonly observed in Japan[24-27]. Moreover, Japan is apparently at a geographical boundary for genotypes B and C, forming a south to north gradient in which genotype C is more frequent in the south of Kyushu, and genotype B is more frequent in the north of Tohoku. Interestingly, however, genotype B is more frequent in Okinawa, the southern-most area of Japan[27]. Our previous epidemiological study of the Japanese HBV genotype distribution showed that 95% of the patients studied had genotype C[24]. Genotype C has been reported to cause more severe liver damage and to have lower rates of HBeAg clearance, which usually indicates cessation of HBV replication and represents a later stage of chronic HBV infection, than genotype B in Japanese patients[24-26]. Accordingly, our results were equivalent in the response to lamivudine to Japanese HBV genotype C patients, although we did not determine the genotyping of our patients.
Another noteworthy finding of our study was that predictive marker of the efficacy to lamivudine and its durability were HBeAg negativity and a low HBV DNA level at baseline. HBV DNA reappears in serum after cessation of lamivudine treatment because HBV replication within the HBV-infected hepatocytes originates primarily from the covalently closed circular DNA (cccDNA) of HBV in the liver. Lamivudine appears to have no effect on the level of cccDNA[28]. Liver injury seems to be particularly severe and rapidly progressive in HBeAg-negative patients, but clinically significant HBV replication persists in them[24]. Most HBeAg-negative chronic hepatitis B patients who are HBV DNA-positive harbor HBV variants with mutations in the precore or core promotor region, which can suppress synthesis of HBeAg[11,26]. The clearance of HBeAg is perhaps a reflection of a loss of the cccDNA pool of HBV in the liver[29]. The great concern of clinicians is that HBeAg negativity and a low HBV DNA level at baseline are significant predictive markers for lamivudine treatment in Japanese patients.
A previous report on Japanese patients showed that the emergence rate of lamivudine-resistant viruses in patients with cirrhosis was higher than those without cirrhosis[28], suggesting that a virological breakthrough appears more frequently in patients with cirrhosis than those without cirrhosis. The present study showed that lamivudine treatment was not so effective or durable in patients with cirrhosis and low platelet counts. Clinicians should always do close monitoring or use other antiviral drugs because hepatitis flare was occasionally severe, especially in patients with cirrhosis. The present study also showed that an early virological response to lamivudine was predictive of both efficacy and durability, but a lack of an early virological response was found to predict a virological breakthrough. A high HBV DNA level reflects a greater pool of virus and a higher rate of virus replication, thereby increasing the likelihood that drug-resistant mutations will be selected. Such an early decrease of viral load after the start of lamivudine might be associated with the lack of viral resistance.
In conclusion, the present study suggests a long-term lamivudine treatment to be safe and to result in the reduction of serum HBV DNA in most Japanese patients with chronic hepatitis B. The efficacy is sustained in patients with HBeAg-negative at baseline, absence of cirrhosis, and a reduction of the HBV DNA level soon after the start of the treatment.
Hironori Ebihara, Kazukuni Kawasaki and Toshihiro Ueda for their advice and help for this study; the following investigators of the KULDS Group were involved in the present study: H. Nakashima, Haradoi Hospital, Fukuoka: N. Kubo, Yagi Hospital, Fukuoka: Y Yokota, Yokota Hospital, Hirokawa, Fukuoka: T. Kuga, and A. Mitsutake, Mitsutake Hospital, Iki, Nagasaki: H. Ohnishi, S. Maeda, and Y. Nakagawa, Yamamoto Surgical Hospital, Imari, Saga: Nagasaki, R. Sugimoto, Harasanshin Hospital, Fukuoka: H. Amagase and S. Tominaga, Mihagino Hospital, Kitakyushu: K. Yanagita, Saiseikai Karatsu Hospital, Karatsu: K. Ogiwara, Kyusyu Rosai Hospital, Kitakyushu: M. Tokumatsu, Saiseikai Fukuoka Hospital, Fukuoka: S. Tabata, Hayashi Hospital, Fukuoka: M. Yokota, National Kyushu Cancer Center, Fukuoka: H. Tanaka, Chihaya Hospital, Fukuoka: S. Nagase, Fukuoka Teishin Hospital, Fukuoka: S. Tsuruta, Nakabaru Hospital, Fukuoka: S. Tada, Moji Rosai Hospital, Kitakyushu: M. Nagano, Kyushu Koseinenkin Hospital, Kitakyushu: M. Honda, Nishi-Fukuoka Hospital, Fukuoka: T. Umeno, Sawara Hospital, Fukuoka: T. Sugimura, National Hospital Organization Fukuoka Higashi Hospital, Fukuoka: S. Ueno, Kitakyushu Municipal Wakamatsu Hospital, Kitakyushu: K. Miki, Kitakyushu Municipal Moji Hospital, Kitakyushu: H. Okubo, Shineikai Hospital, Kitakyushu: H. Fujimoto, Mitsubishikagaku Hospital, Kitakyushu: N. Higuchi, Shin-Nakama Hospital, Kitakyushu: S. Shigematsu, Kouseikan Hospital, Saga: N. Higashi, National Hospital Organization Beppu Hospital, Beppu, Japan.
S- Editor Kumar M, Guo SY and Pan BR L- Editor Elsevier HK E- Editor Bi L
| 1. | Lee WM. Hepatitis B virus infection. N Engl J Med. 1997;337:1733-1745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1728] [Cited by in RCA: 1712] [Article Influence: 61.1] [Reference Citation Analysis (0)] |
| 2. | Furusyo N, Hayashi J, Sawayama Y, Kishihara Y, Kashiwagi S. Hepatitis B surface antigen disappearance and hepatitis B surface antigen subtype: a prospective, long-term, follow-up study of Japanese residents of Okinawa, Japan with chronic hepatitis B virus infection. Am J Trop Med Hyg. 1999;60:616-622. [PubMed] |
| 3. | Lee JS, Thorgeirsson SS. Genome-scale profiling of gene expression in hepatocellular carcinoma: classification, survival prediction, and identification of therapeutic targets. Gastroenterology. 2004;127:S51-S55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 136] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 4. | Hoofnagle JH, di Bisceglie AM. The treatment of chronic viral hepatitis. N Engl J Med. 1997;336:347-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 715] [Cited by in RCA: 681] [Article Influence: 24.3] [Reference Citation Analysis (1)] |
| 5. | Dienstag JL, Perrillo RP, Schiff ER, Bartholomew M, Vicary C, Rubin M. A preliminary trial of lamivudine for chronic hepatitis B infection. N Engl J Med. 1995;333:1657-1661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 620] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 6. | Lai CL, Chien RN, Leung NW, Chang TT, Guan R, Tai DI, Ng KY, Wu PC, Dent JC, Barber J. A one-year trial of lamivudine for chronic hepatitis B. Asia Hepatitis Lamivudine Study Group. N Engl J Med. 1998;339:61-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1381] [Cited by in RCA: 1347] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 7. | Tassopoulos NC, Volpes R, Pastore G, Heathcote J, Buti M, Goldin RD, Hawley S, Barber J, Condreay L, Gray DF. Efficacy of lamivudine in patients with hepatitis B e antigen-negative/hepatitis B virus DNA-positive (precore mutant) chronic hepatitis B. Lamivudine Precore Mutant Study Group. Hepatology. 1999;29:889-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 353] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 8. | Perrillo R, Rakela J, Dienstag J, Levy G, Martin P, Wright T, Caldwell S, Schiff E, Gish R, Villeneuve JP. Multicenter study of lamivudine therapy for hepatitis B after liver transplantation. Lamivudine Transplant Group. Hepatology. 1999;29:1581-1586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 201] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 9. | Doong SL, Tsai CH, Schinazi RF, Liotta DC, Cheng YC. Inhibition of the replication of hepatitis B virus in vitro by 2',3'-dideoxy-3'-thiacytidine and related analogues. Proc Natl Acad Sci USA. 1991;88:8495-8499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 394] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 10. | Honkoop P, Niesters HG, de Man RA, Osterhaus AD, Schalm SW. Lamivudine resistance in immunocompetent chronic hepatitis B. Incidence and patterns. J Hepatol. 1997;26:1393-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 216] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 11. | Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology. 2001;34:1225-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 642] [Cited by in RCA: 639] [Article Influence: 26.6] [Reference Citation Analysis (1)] |
| 12. | Leung NW, Lai CL, Chang TT, Guan R, Lee CM, Ng KY, Lim SG, Wu PC, Dent JC, Edmundson S. Extended lamivudine treatment in patients with chronic hepatitis B enhances hepatitis B e antigen seroconversion rates: results after 3 years of therapy. Hepatology. 2001;33:1527-1532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 487] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 13. | Oh JM, Kyun J, Cho SW. Long-term lamivudine therapy for chronic hepatitis B in patients with and without cirrhosis. Pharmacotherapy. 2002;22:1226-1234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Lok AS, Heathcote EJ, Hoofnagle JH. Management of hepatitis B: 2000--summary of a workshop. Gastroenterology. 2001;120:1828-1853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 540] [Cited by in RCA: 513] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 15. | Niederau C, Heintges T, Lange S, Goldmann G, Niederau CM, Mohr L, Häussinger D. Long-term follow-up of HBeAg-positive patients treated with interferon alfa for chronic hepatitis B. N Engl J Med. 1996;334:1422-1427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 609] [Cited by in RCA: 574] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 16. | Liaw YF, Leung NW, Chang TT, Guan R, Tai DI, Ng KY, Chien RN, Dent J, Roman L, Edmundson S. Effects of extended lamivudine therapy in Asian patients with chronic hepatitis B. Asia Hepatitis Lamivudine Study Group. Gastroenterology. 2000;119:172-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 532] [Cited by in RCA: 519] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 17. | Chang TT, Lai CL, Chien RN, Guan R, Lim SG, Lee CM, Ng KY, Nicholls GJ, Dent JC, Leung NW. Four years of lamivudine treatment in Chinese patients with chronic hepatitis B. J Gastroenterol Hepatol. 2004;19:1276-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 196] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 18. | Lau DT, Everhart J, Kleiner DE, Park Y, Vergalla J, Schmid P, Hoofnagle JH. Long-term follow-up of patients with chronic hepatitis B treated with interferon alfa. Gastroenterology. 1997;113:1660-1667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 212] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 19. | Lok AS. Prevention of hepatitis B virus-related hepatocellular carcinoma. Gastroenterology. 2004;127:S303-S309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 101] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 20. | Honkoop P, de Man RA, Niesters HG, Zondervan PE, Schalm SW. Acute exacerbation of chronic hepatitis B virus infection after withdrawal of lamivudine therapy. Hepatology. 2000;32:635-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 170] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 21. | Okamoto H, Tsuda F, Sakugawa H, Sastrosoewignjo RI, Imai M, Miyakawa Y, Mayumi M. Typing hepatitis B virus by homology in nucleotide sequence: comparison of surface antigen subtypes. J Gen Virol. 1988;69:2575-2583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 725] [Cited by in RCA: 769] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 22. | Norder H, Couroucé AM, Magnius LO. Complete genomes, phylogenetic relatedness, and structural proteins of six strains of the hepatitis B virus, four of which represent two new genotypes. Virology. 1994;198:489-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 556] [Cited by in RCA: 587] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 23. | Mayerat C, Mantegani A, Frei PC. Does hepatitis B virus (HBV) genotype influence the clinical outcome of HBV infection. J Viral Hepat. 1999;6:299-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 201] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 24. | Furusyo N, Nakashima H, Kashiwagi K, Kubo N, Hayashida K, Usuda S, Mishiro S, Kashiwagi S, Hayashi J. Clinical outcomes of hepatitis B virus (HBV) genotypes B and C in Japanese patients with chronic HBV infection. Am J Trop Med Hyg. 2002;67:151-157. [PubMed] |
| 25. | Furusyo N, Kubo N, Nakashima H, Kashiwagi K, Hayashi J. Relationship of genotype rather than race to hepatitis B virus pathogenicity: a study of Japanese and Solomon Islanders. Am J Trop Med Hyg. 2004;70:571-575. [PubMed] |
| 26. | Nakashima H, Furusyo N, Kubo N, Kashiwagi K, Etoh Y, Kashiwagi S, Hayashi J. Double point mutation in the core promoter region of hepatitis B virus (HBV) genotype C may be related to liver deterioration in patients with chronic HBV infection. J Gastroenterol Hepatol. 2004;19:541-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Usuda S, Okamoto H, Iwanari H, Baba K, Tsuda F, Miyakawa Y, Mayumi M. Serological detection of hepatitis B virus genotypes by ELISA with monoclonal antibodies to type-specific epitopes in the preS2-region product. J Virol Methods. 1999;80:97-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 224] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 28. | Ooga H, Suzuki F, Tsubota A, Arase Y, Suzuki Y, Akuta N, Sezaki H, Hosaka T, Someya T, Kobayashi M. Efficacy of lamivudine treatment in Japanese patients with hepatitis B virus-related cirrhosis. J Gastroenterol. 2004;39:1078-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 29. | Park NH, Shin JW, Park JH, Bang SJ, Kim DH, Joo KR, Kim DH. Monitoring of HBeAg levels may help to predict the outcomes of lamivudine therapy for HBeAg positive chronic hepatitis B. J Viral Hepat. 2005;12:216-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |