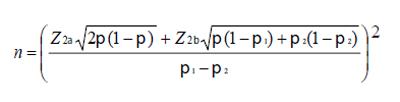Published online Oct 21, 2006. doi: 10.3748/wjg.v12.i39.6366
Revised: January 28, 2006
Accepted: May 22, 2006
Published online: October 21, 2006
AIM: To compare the efficacy and safety of five days apostrophe therapy of mebendazole (MBZ) versus quinacrine (QC) on human giardiasis in children.
METHODS: A clinical trial was carried out in paediatric patients (aged 5-15 years) with confirmed symptomatic G. duodenalis mono-infection. Patients were randomly assigned to receive either MBZ [200 mg taken three times per day (TID) (n = 61)] or QC [2 mg/kg bodyweight tid (n = 61)], both for five days. Follow-up faecal samples were obtained at 3, 5 and 7 d after the end of the treatment.
RESULTS: Although the frequency of cure was higher for QC (83.6%) than for MBZ (78.7%), the difference was not statistically significant (P > 0.05). Adverse events were reported more in the QC group (P < 0.05), all of them transient and self-limiting.
CONCLUSION: Despite final cure rates ocurring lower than expected, the overall results of this study reconfirmed the efficacy of MBZ in giardiasis and also indicate that, although comparable to QC, at least in this setting the 5 d course of MBZ did not appear to improve the cure rates in this intestinal parasitic infection.
- Citation: Cañete R, Escobedo AA, González ME, Almirall P. Randomized clinical study of five days apostrophe therapy with mebendazole compared to quinacrine in the treatment of symptomatic giardiasis in children. World J Gastroenterol 2006; 12(39): 6366-6370
- URL: https://www.wjgnet.com/1007-9327/full/v12/i39/6366.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i39.6366
Giardiasis is one of the commonest intestinal parasitic diseases diagnosed worldwide. Its clinical presentation is highly variable, most of the time it is characterized by mild and self-limiting signs and symptoms. However, it is not unusual that in some cases, this disease can become a significant cause of morbidity, resulting in malabsorption of fats, vitamins and lactose with serious consequences, particularly in children, causing failure-to-thrive syndrome[1,2].
Classically, 5-nitroimidazole compounds have been accepted for decades the world over as the “gold standard” for treatment of patients with giardiasis. However, there is an increasing number of treatment failures reported in the literature, requiring repeated courses of the same drug, change to other compounds or make a combination of two anti-Giardia drugs for therapy[3,4]. Consequently, newer and older drugs, such as nitazoxanide[5,6] and chloroquine[7] respectively, have been proposed as alternatives for the treatment of this intestinal infection.
In recent years, chemotherapy with mebendazole (MBZ), the benzimidazole-carbamate compound that has been extensively used in the treatment of helmintic infections, has also demonstrated in vitro activity against Giardia duodenalis (G. duodenalis)[8-14], the causative agent of human giardiasis. Clinical experience has also shown therapeutic benefits for the use of MBZ in this infection[15-17], although it is usually accepted, the optimal dosage and duration of the treatment remain to be determined. For this reason, in the present study it was decided to evaluate the efficacy and safety of a five-day regimen of MBZ versus quinacrine (QC) in the treatment of giardiasis in a group of Cuban paediatric patients.
This study was carried out at the Gastroenterology institute in Havana, Cuba. In order to recruit a sufficient number of patients for this trial, all the specialists of the institution were invited to refer their paediatric patients (aged 5-15 years) who were received seeking treatment for symptomatic acute G. duodenalis infection with or without diarrhoea from May to December, 2003. To be eligible for the study a child had to have mono-infection with G. duodenalis (proven by microscopic examination of faecal samples, as wet mounts and/or after Ritchie concentration[18]). Patients were excluded from the study if any of the following conditions were present: (1) known history of sensitivity to any of benzimidazole compounds or QC, (2) had received any antiparasitic chemotherapy in the preceding 4 wk, (3) diseases other than giardiasis. Also were excluded those who were unlikely to attend follow-up examinations.
The protocol was approved by the Research and Ethics committee of the institute. Parents or legal guardians of each child were fully informed about the aim of the study, the characteristics of the drugs under investigation and they were told that his/her childs participation was optional. Written informed consent was obtained from them prior to trial enrollment.
The sample size for each treatment group (n) needed to ensure sufficient statistical power (80%) to reject the null hypothesis that MBZ and QC are not equally effective (in terms of a parasitological cure) with a significant level of 5%, was calculated according to Armitage and Berry[19]. The following equation was used:
Math 1
where:
π1: denotes the proportion of population cured with standard treatment.
π2: denotes the proportion of population cured with the assayed treatment.
Z2α = 1.96
Z2β = 0.842
One hundred and twenty two children were required (i.e. 61 in each treatment arm).
Patients who (a) were eligible, (b) met none of the exclusion criteria, and (c) one of their parents or legal guardians had given written informed consent for the trial, were included in the study. A random-number table was used to allocate each of these 122 children to receive either MBZ (Reynaldo Gutierrez Pharmaceutical, Havana) 200 mg thrice a day or QC (Reynaldo Gutierrez Pharmaceutical, Havana) at 2 mg/kg bodyweight thrice daily, both for 5 d. The drugs were provided at no charge.
A detailed history was taken from the accompanying parent or legal guardian; a standardized questionnaire was used to record clinical signs and symptoms before starting treatment and at the end. Also, a physical examination was carried out, and each child was weighed.
Comprehensive oral instructions were given to all children and parents or legal guardians accompanying them in an attempt to maintain a high level of compliance with the study plan, including the administration of the drug and the importance of good hygiene and of measures they could take to reduce the risk of giardial infection in the future.
The evaluation of efficacy of the chemotherapy was based on parasitological response to therapy assessed by the same laboratory tests that were done initially. Parents or legal guardians of each child were asked to provide three faecal samples on d 3, 5, and 7 after treatment completion in order to avoid including the possibility of re-infection, considering it is a very frequent phenomenon in children, mainly in developing countries. Also, they were encouraged to return to the clinic at any time, if they considered that his or her child was ill. A child was only considered to be cured, if no Giardia trophozoites or cysts could be found in any of the three post-treatment faecal specimens.
Irrespective of their causal relationship to study treatment, details of all clinical adverse events reported spontaneously at any time during the trial, regardless of their relationship to the study drugs and those elicited by the investigators with no leading questions in each of the clinic visits scheduled, were taken into account. Adverse event was defined as the development of any sign or symptoms that did not exist before or which became more serious following the commencement of the treatment. Serious adverse events were defined as death, any life-threatening, disabling or incapacitating events, or those requiring hospitalization.
The data on the parasitological response and adverse events were taken on pre-designed record forms and subsequently analysed to determine the frequency of each response/effect. The statistical significance of differences between mean values was determined using the Student’s t-test. Where appropriate Fisher exact test (χ2 test) was used to establish the significance of differences in proportions.
All 122 paediatric patients who began treatment completed this study and were included in the statistical analysis. Characteristics of the treatment groups at the entry with regard to sex and age are presented (Table 1). There were no significant-between the groups- differences in demographics (P > 0.05).
| MBZ group | QC group | |
| n = 61 | n = 61 | |
| Sex | ||
| Male: n (%) | 28 (45.9) | 26 (42.6) |
| Female: n (%) | 33 (54.1) | 35 (57.3) |
| Age (yr) | ||
| Mean (range) | 8.2 (5-15) | 9.7 (5-15) |
The efficacy did not differ significantly between the two groups (P > 0.05), although it was slightly lower in the MBZ group [48 out of 61 (78.7%)] than that seen in the group treated with QC (83.6%) (Table 2).
| MBZ group | QC group | |
| n = 61 | n = 61 | |
| Cure rate | 48 (78.7%) | 51 (83.6%) |
| Any adverse event | 14 (22.9%) | 36 (59%) |
| Abdominal pain | 11 (18 %) | 10 (16.3%) |
| Vomiting | 3 (4.9%) | 14 (22.9%) |
| Nausea | 3 (4.9%) | 14 (22.9%) |
| Headache | 1 (1.6%) | 11 (18%) |
| Yellowish coloration of the skin | - | 15 (24.6%) |
Adverse events recorded with any treatment are also shown in Table 2. The therapy with the drugs was well tolerated and did not produce adverse events that warranted discontinuation of the study medication; in fact, no patients had to stop treatment because of potentially drug-related adverse events. Of the 122 patients studied, 72 (59%) had no adverse events, but 50 [14 in the MBZ group (22.9%) and 36 (59%) in the group treated with QC] reported at least one such event, none of them unexpected. Nausea, vomiting, headache and yellow discoloration were reported statistically significant by the QC group (P < 0.05). All adverse events were graded as mild, transient in nature, and did not require administration of drugs for relief or hospitalization. Except for yellow discoloration which lasted longer, the rest of the clinical adverse events usually resolved within 1-2 d and any of them were severe enough to interfere with activities of daily living.
Treatment failures with the currently available drugs used to treat giardiasis have provided a continuous stimulus to search for other therapeutic alternatives. MBZ has been one of the drugs proposed in the treatment of this infection due to the observations of Hutchison et al[20] who during their investigation of the activity of this drug against intestinal nematodes realized that some cases of Giardia infection could also be cured. Lately, although in vitro studies not only confirmed the effect of MBZ on Giardia trophozoites, but have also served to clarify its activity against this protozoan, a number of clinical studies have been performed in adults and children comparing this drug with the currently available antigiardial drugs. This could be, in part, because giardiasis as a new indication of MBZ is still a topic of discussion due to the contradictory clinical reports concerning its efficacy. Thus, while most of the published clinical data documented the effect of this drug in Giardia infections, in contrast there are some reports that show the failure of this drug to clear their patients´ symptoms or stop the patients from excreting Giardia cysts using MBZ for 1 and 5 d, respectively[21,22]; and interestingly, Rousham[23] noticed that during a deworming study in northern Bangladesh, the prevalence of G. duodenalis increased significantly among children receiving periodic treatment with MBZ; for this reason there is a continuous necessity to evaluate treatment dosage and regimens with this drug.
The present study again confirms previous findings that MBZ is an attractive and efficacious agent against Giardia infections because it can be easily administered and has not been associated with serious adverse events. In a previous trial of MBZ, Bulut et al[24] used this drug at 100 mg thrice for 1 d and for 7 d and obtained responses of 41.7% and 58.3%, respectively. Other studies have reported higher parasitological response rates e.g., Rodriguez-Garcia et al[5], found that treatment with administered 100 mg of MBZ every 12 h, for 3 d achieved a cure rate of 80.4% that was comparable to 100 mg of nitazoxanide administered in the same way. In a closely related trial, similar outcomes were obtained by Sadjjadi et al[16], who used 200 mg of MBZ thrice daily for 5 d -which resulted in somewhat higher cure rates [43 out of 50 (86%)] than that seen in the present study- and found a frequency comparable to that obtained by using a 7-d course of metronidazole.
Based on the experience of Sadjjadi et al[16] and on a previous study carried out in Cuba in paediatric patients for 3 d where the cure rates obtained were 78.1%[17], we decided to treat patients in this current study with a 5-d course of MBZ at 200 mg thrice a day in order to see if the response rate could be improved after this prolonged course; however, no additional benefit in the efficacy rate could be demonstrated.
Contrary to what was expected, it was noted that QC resulted in considerably lower cure rates than that which have been previously reported by other authors. This drug was widely used for the chemosuppression of malaria and used to be the front line drug for the treatment of adult giardiasis, but it was superseded for these purposes by chloroquine and metronidazole, respectively. QC has been regarded the most efficacious drug of any of the anti-Giardia therapeutics by some researchers[25] because its clinical efficacy in giardiasis has been found over 90%. This drug also has the benefits that even in patients with severe diarrhoea its intestinal absorption is not interfered[26] with and it has the additional epidemiological advantage that it could also kill cysts[27]. However, this drug has the potential of adverse events, including yellow discoloration of the skin, bitter taste, nausea and vomiting, and it has also been reported in toxic psychosis[28]. For these reasons, at this moment this old drug might experience a revival in the clinical management of patients where other drugs have failed (to whom it has almost wholly been reserved). Nevertheless, clinical reports in which Giardia has not been eliminated despite one or more appropriate courses with QC have been published[3,29]. Additionally, in one study resistance against QC could be induced in Giardia laboratory stocks[30].
In the present study, no new or previously undescribed adverse events occurred. Both drugs were well tolerated, resulting in good patient compliance. In no case, adverse events observed lead to discontinuation of the drugs. The adverse events observed were all mild, transient in nature and self-limiting; all generally occurred at similar frequencies to those observed in previous trials with the same drugs. In general, MBZ is very well tolerated which could be due, in part, to the advantage of being hardly absorbed from the gut (no more than 20% of the dose, even after a fat rich meal)[31].
One possible weakness in the current study was that for practical reasons it was conducted in an open fashion. As the two drug treatments look very different and the number of tablets to take daily varied it was impossible to make the study blind. Certainly, in the market it would have had been possible to obtain placebos for the two drugs but this would have been costly for the study. This could be a limitation and consequently, despite well-defined pre-study criteria for evaluating efficacy and safety, evaluation of the treatment response and possible cause of adverse events could have been somewhat biased; but it could not have influenced the major result (eradication of Giardia infection) because the efficacy analysis was done by the laboratory department where those checking post-treatment faecal samples were unaware of the treatment allocation and had no clinical involvement with the paediatric patients or their parents.
For us, this study has the strength that in many countries MBZ is not considered even a major alternative treatment for giardiasis; therefore, it is useful information that there is a therapeutically effective high dose used daily-at least three times the usual dose for helminthic infections caused by hookworms and six times that used for enterobiasis. Additionally, there have been several studies of MBZ for giardiasis, but it is the first to compare with QC which has permitted evaluation and update of the current efficacy of QC in Cuban children with giardiasis.
Where does the present data lead us with the use of MBZ in giardiasis That is the question that could have been asked to answer given the current context. We shall approach the problem by offering a series of related conclusions: clearly, our study adds support to the observation that this drug has a place as an alternative treatment of giardiasis in children when (a) other first-line drugs have failed, (b) in areas where this infection and other sensitive organisms, e.g., intestinal nematodes, are prevalent, (c) in patients with a history of known sensitivity to any of the currently antigiardial compounds, and (d) possibly, as adjunctive therapy in combination with other available antigiardial drugs in order to offer potentially higher cure rates.
Taking into account that the ideal antiparasitic agents would be efficacious, easily dosed and administered, inexpensive, and with few adverse effects, in view of the above-given data for MBZ and QC, neither would be first line drugs, but this paper may help to inform about choices for those people where other currently recommended drugs have failed. MBZ belongs to a chemical family that differs from 5-nitroimidazoles, acts against Giardia duodenalis by a different mechanism, and has a different safety profile. In the present study it was at least as effective as, and better tolerated than QC. All of these advantages should not be forgotten when we are treating giardiasis, but the benefits of extending treatment from three to five consecutive days were not apparent, at least in this setting.
S- Editor Wang J L- Editor Alpini GD E- Editor Ma WH
| 1. | Adam RD. Biology of Giardia lamblia. Clin Microbiol Rev. 2001;14:447-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 875] [Cited by in RCA: 795] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 2. | Ortega YR, Adam RD. Giardia: overview and update. Clin Infect Dis. 1997;25:545-59; quiz 550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 133] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 3. | Taylor GD, Wenman WM, Tyrrell DL. Combined metronidazole and quinacrine hydrochloride therapy for chronic giardiasis. CMAJ. 1987;136:1179-1180. [PubMed] |
| 4. | Nash TE, Ohl CA, Thomas E, Subramanian G, Keiser P, Moore TA. Treatment of patients with refractory giardiasis. Clin Infect Dis. 2001;33:22-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 87] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 5. | Rodríguez-García R, Rodríguez-Guzmán LM, Cruz del Castillo AH. [Effectiveness and safety of mebendazole compared to nitazoxanide in the treatment of Giardia lamblia in children]. Rev Gastroenterol Mex. 1999;64:122-126. [PubMed] |
| 6. | Fox LM, Saravolatz LD. Nitazoxanide: a new thiazolide antiparasitic agent. Clin Infect Dis. 2005;40:1173-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 289] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 7. | Escobedo AA, Núñez FA, Moreira I, Vega E, Pareja A, Almirall P. Comparison of chloroquine, albendazole and tinidazole in the treatment of children with giardiasis. Ann Trop Med Parasitol. 2003;97:367-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Edlind TD, Hang TL, Chakraborty PR. Activity of the anthelmintic benzimidazoles against Giardia lamblia in vitro. J Infect Dis. 1990;162:1408-1411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 76] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 9. | Katelaris PH, Naeem A, Farthing MJ. Activity of metronidazole, azithromycin and three benzimidazoles on Giardia lamblia growth and attachment to a human intestinal cell line. Aliment Pharmacol Ther. 1994;8:187-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Cedillo-Rivera R, Muñoz O. In-vitro susceptibility of Giardia lamblia to albendazole, mebendazole and other chemotherapeutic agents. J Med Microbiol. 1992;37:221-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 79] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Morgan UM, Reynoldson JA, Thompson RC. Activities of several benzimidazoles and tubulin inhibitors against Giardia spp. in vitro. Antimicrob Agents Chemother. 1993;37:328-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 58] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Chavez B, Cedillo-Rivera R, Martinez-Palomo A. Giardia lamblia: ultrastructural study of the in vitro effect of benzimidazoles. J Protozool. 1992;39:510-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 66] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Katiyar SK, Gordon VR, McLaughlin GL, Edlind TD. Antiprotozoal activities of benzimidazoles and correlations with beta-tubulin sequence. Antimicrob Agents Chemother. 1994;38:2086-2090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 139] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 14. | MacDonald LM, Armson A, Thompson AR, Reynoldson JA. Characterisation of benzimidazole binding with recombinant tubulin from Giardia duodenalis, Encephalitozoon intestinalis, and Cryptosporidium parvum. Mol Biochem Parasitol. 2004;138:89-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 67] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | al-Waili NS, al-Waili BH, Saloom KY. Therapeutic use of mebendazole in giardial infections. Trans R Soc Trop Med Hyg. 1988;82:438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Sadjjadi SM, Alborzi AW, Mostovfi H. Comparative clinical trial of mebendazole and metronidazole in giardiasis of children. J Trop Pediatr. 2001;47:176-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Escobedo AA, Cañete R, Gonzalez ME, Pareja A, Cimerman S, Almirall P. A randomized trial comparing mebendazole and secnidazole for the treatment of giardiasis. Ann Trop Med Parasitol. 2003;97:499-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | García LS, Bruckner DA. Diagnostic Medical Parasitology. Macroscopic and Microscopic Examination of fecal specimens. American Society for Microbiology. Washington DC. 1993;501-540. |
| 19. | Armitage P, Berry G. Statistical Methods in Medical Research. 2nd ed. Oxford: Blackwell Scientific 1987; . |
| 20. | Hutchison JG, Johnston NM, Plevey MV, Thangkhiew I, Aidney C. Clinical trial of mebendazole, a broad-spectrum anthelminthic. Br Med J. 1975;2:309-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Gascon J, Moreno A, Valls ME, Miró JM, Corachán M. Failure of mebendazole treatment in Giardia lamblia infection. Trans R Soc Trop Med Hyg. 1989;83:647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | di Martino L, Nocerino A, Mantovani MP. Mebendazole in giardial infections: confirmation of the failure of this treatment. Trans R Soc Trop Med Hyg. 1991;85:557-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Rousham EK. An increase in Giardia duodenalis infection among children receiving periodic Anthelmintic treatment in Bangladesh. J Trop Pediatr. 1994;40:329-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Bulut BU, Gülnar SB, Aysev D. Alternative treatment protocols in giardiasis: a pilot study. Scand J Infect Dis. 1996;28:493-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Wolfe MS. Giardiasis. Clin Microbiol Rev. 1992;5:93-100. [PubMed] |
| 26. | Gardner TB, Hill DR. Treatment of giardiasis. Clin Microbiol Rev. 2001;14:114-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 337] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 27. | Gillin FD, Diamond LS. Inhibition of clonal growth of Giardia lamblia and Entamoeba histolytica by metronidazole, quinacrine, and other antimicrobial agents. J Antimicrob Chemother. 1981;8:305-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 45] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Genel F, Erermis S, Aksu G, Ozturk C, Kutukculer N. Quinacrine-induced psychiatric disturbances in a child with common variable immunodeficiency and chronic giardiasis. Hum Psychopharmacol. 2002;17:357-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 29. | Smith PD, Gillin FD, Spira WM, Nash TE. Chronic giardiasis: studies on drug sensitivity, toxin production, and host immune response. Gastroenterology. 1982;83:797-803. [PubMed] |
| 30. | Upcroft JA, Campbell RW, Upcroft P. Quinacrine-resistant Giardia duodenalis. Parasitology. 1996;112:309-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |