Published online Oct 21, 2006. doi: 10.3748/wjg.v12.i39.6349
Revised: November 28, 2005
Accepted: August 30, 2006
Published online: October 21, 2006
AIM: To evaluate the functional aspect of esophageal motility in healthy subjects and in patients who were referred for esophageal function testing using multichannel intraluminal impedance-esophageal manometry (MII-EM), and to assess the clinical utility of MII-EM.
METHODS: From September 2003 to January 2004, we performed the MII-EM on healthy volunteers and all the patients who were referred for esophageal function testing. Each patient received 10 liquid and 10 viscous swallows. We analyzed the results, the impedance and the manometric findings. Some of the subjects had additional ambulatory 24-h pH study performed to diagnose gastroesophageal reflux disease (GERD).
RESULTS: Among 89 studied subjects, the MII-EM findings showed normal esophageal motility in 50 (56.17%), ineffective esophageal motility (IEM) in 17 (19.10%), nutcracker esophagus in 7 (7.86%), achalasia in 4 (4.49%), and scleroderma esophagus in 11 (12.35%) cases. The completeness and the speed of bolus transit were in the order of nutcracker esophagus, normal manometry and IEM. Some of the swallows showing normal manometry and IEM had incomplete transit. In the achalasia and scleroderma esophagus, almost all the swallows had incomplete transit. The body amplitudes were higher for the swallows with complete transit than for the swallows with incomplete transit. There was not a significant difference in the manometric and impedance findings between the subjects with and without GERD.
CONCLUSION: MII-EM is a useful tool in assessing the esophageal function in the patients having esophageal motility abnormality. The primary factors influencing the bolus transit are the amplitude of the esophageal body and normal peristalsis.
- Citation: Cho YK, Choi MG, Park JM, Oh JH, Paik CN, Lee JW, Lee IS, Kim SW, Chung IS. Evaluation of esophageal function in patients with esophageal motor abnormalities using multichannel intraluminal impedance esophageal manometry. World J Gastroenterol 2006; 12(39): 6349-6354
- URL: https://www.wjgnet.com/1007-9327/full/v12/i39/6349.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i39.6349
Esophageal manometry has been considered the “gold standard” test for the evaluation of esophageal motility. Esophageal manometry allows physicians to assess peristalsis by using informations about the shape, amplitude and duration of the esophageal contraction, but it does not offer direct information about bolus transit, while barium study and single photon emission computed tomography visualize the bolus transit and offer anatomical information, but these techniques have the disadvantage of radiation exposure[1].
The principles of impedance testing are based on measuring the differences in resistance to an alternating current that passes through the intraluminal contents. Impedance testing can detect and quantify bolus movement[2-4]. The ability of impedance testing to detect bolus transit was validated with video-fluoroscopy in normal subjects[5,6]. Combined multichannel intraluminal impedance-esophageal manometry (MII-EM) evaluates the functional aspects of esophageal contractions by simultaneously measuring bolus transit and esophageal contraction. Generally, the findings of esophageal manometry correlate with the symptoms or clinical progress in achalasia and diffuse esophageal spasm, but their findings do not always correlate in nutcracker esophagus and ineffective esophageal motility (IEM). The assessment of bolus transit and esophageal function may help the physician to understand the clinical progress of a patient with esophageal disease. The aim of this study was to evaluate the functional aspects of esophageal contraction in patients having diverse esophageal motility abnormalities by using MII-EM, and to investigate its clinical utility.
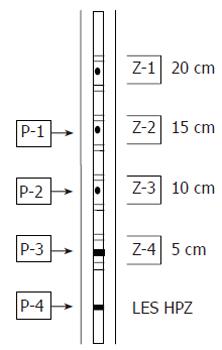
From September 2003 to January 2004, all the subjects who were referred for esophageal function testing and the healthy volunteers had esophageal function testing performed using MII-EM technology at Kangnam St. Mary’s hospital, the Catholic university. The volunteers did not have any esophageal symptoms, as was investigated by an esophageal symptoms questionnaire, and they also did not have any gastrointestinal motility disorders that would influence esophageal motility, as was ascertained by physical examination and history taking. The MII-EM catheter (Sandhill EFT catheter; Sandhill Scientific Inc. Highland Ranch, CO.) was inserted transnasally into the esophagus. The 4.5-mm diameter catheter design had two circumferential solid-state pressure sensors at 5 cm and 10 cm from the tip, and it had two unidirectional pressure sensors at 15 cm and 20 cm from the tip. The impedance measuring segments consisted of pairs of metal rings placed 2 cm apart and they were centered at 10, 15, 20 and 25 cm from the tip. Intraesophageal pressure sensors and impedance measuring segments were thus located at 5, 10, 15 and 20 cm above the lower esophageal sphincter (LES), respectively (Figure 1). The LES was identified using the stationary pull-through method. The esophageal function test was performed after the subjects had rested for 10 min. With the patients in a sitting position, they were given 10 swallows of 5 cm3 normal saline and 10 swallows of 5 cm3 viscous material at 20-30 s apart. Normal saline was used instead of water since it has a standardized ionic concentration and provides for better impedance changes. The viscous material was manufactured as a food substance, and its known, standardized impedance value was provided by Sandhill Scientific Co.
Manometry: The swallows were manometrically classified as (1) normal if the contraction amplitudes at 5 and 10 cm above the LES were each greater than or equal to 30 mmHg, and the distal onset velocity was less than 8 cm/s, (2) ineffective if either of the contraction amplitudes at 5 and 10 cm above the LES was less than 30 mmHg, (3) simultaneous if the contraction amplitudes at 5 and 10 cm above LES were each greater than or equal to 30 mmHg at the site, and the distal onset velocity was greater than 8 cm/s.
The manometric parameters used to characterize the swallows included (1) the contraction amplitude at 5 and 10 cm above the LES, (2) the distal esophageal amplitude as the average of the contraction amplitudes at 5 and 10 cm above the LES, and (3) the onset velocity of the esophageal contractions in the distal part of the esophagus. The mid-respiratory resting pressure and the LES residual pressure during swallows were used to assess the LES function.
Impedance: In the impedance curve, the bolus entry at each level was determined as the 50% point between the 3-s pre-swallow impedance baseline and the impedance nadir during the presence of the bolus, and the bolus exit was determined as the return to this 50% bolus point on the impedance recovery curve.
The swallows were classified by MII as showing (1) complete bolus transit, if the bolus entry occurred at the most proximal site (20 cm above LES) and the bolus exit points were recorded at all three distal impedance measuring sites (15, 10 and 5 cm above LES), (2) incomplete bolus transit, if the bolus exit points were not identified at any one of the three distal impedance measuring sites. We calculated the complete bolus transit rate for the liquid and viscous swallows.
Impedance parameters included (1) the total bolus transit time (TBTT) as the time that elapsed between bolus entry at 20 cm above the LES and bolus exit at 5 cm above the LES, (2) the complete bolus transit rate, and (3) the baseline impedance during the resting state. To assess the baseline impedance value, a pair of cursors (the time difference between the cursors was about 4 s) were placed on one channel about 2-3 s before the onset of the impedance changes related to the arrival of a bolus front. The maximal and minimal values between the cursors were subsequently determined by computer analysis. The baseline impedance was defined as the mean of these values.
Evaluation of esophageal motility and function: The diagnoses of manometric motility abnormalities were established from 10 liquid swallows with using the criteria published by Spechler and Castell[7]. Achalasia was defined by the absence of esophageal body peristalsis and, if present, a poorly relaxing LES. Scleroderma esophagus was defined based on an appropriate clinical diagnosis and confirmed by the presence of low amplitude contractions in the distal esophagus with or without a low LES pressure. Diffuse esophageal spasm was defined as 20% or more simultaneous contractions. IEM was defined as 30% or more swallows with a contraction amplitude less than 30 mmHg in either of the two distal sites located at 5 and 10 cm above the LES. Nutcracker esophagus was defined as normal peristalsis of the esophageal body with the average distal esophageal amplitude exceeding 180 mmHg. Poorly relaxing LES was defined as the average LES residual pressure exceeding 8 mmHg and this was associated with normal esophageal body contractions. Hypertensive LES was defined as the LES resting pressure exceeding 45 mmHg with normal esophageal body contractions. Hypotensive LES was defined as the LES resting pressure below 10 mmHg with normal esophageal body contractions. Normal esophageal manometry was defined as not more than 20% ineffective swallows and not more than 10% simultaneous swallows with a distal esophageal amplitude < 180 mmHg and with normal LES resting and residual pressures. For the patients having both esophageal body and LES abnormalities, the final diagnosis was based on the esophageal body findings.
The overall diagnosis of esophageal transit abnormalities was defined as abnormal liquid transit if more than 20% of the liquid swallows had incomplete bolus transit and there was abnormal viscous transit if more than 30% of the viscous swallows had incomplete bolus transit.
Descriptive statistics were used to describe the manometric and impedance findings. Analysis of variance (ANOVA) was used to evaluate the differences of impedance parameters by the manometric diagnosis. Unpaired t-test was used to compare the differences of the distal esophageal amplitudes between swallows with and without complete transit. Unpaired t-test was also used in the comparison of the manometric and impedance parameters between the patients with and without gastroesophageal reflux. A P value less than 0.05 was considered statistically significant.
Using combined MII-EM, 89 subjects including 26 healthy volunteers (27 males and 62 females, mean age: 41.4 years), underwent esophageal function testing. While all the subjects received liquid swallows, 10 subjects did not receive the viscous swallows. The primary symptoms for which the subjects received esophageal function testing were: ENT symptoms, such as throat discomfort or a globus sense in 26 subjects, chronic cough in 5 subjects, heartburn in 16 subjects (including 6 scleroderma patients), dysphagia in 4 subjects, other symptoms in 4 subjects and there were asymptomatic 8 scleroderma patients in order to evaluate the esophageal involvement of scleroderma. In 37 subjects, esophageal function testing was performed prior to ambulatory 24-h pH monitoring, which was done to diagnose gastroesophageal reflux disease (GERD).
Based on the aforementioned manometric criteria of liquid swallows, 50 (56.17%) patients had normal esophageal manometry, 17 (19.10%) patients had IEM, 7 (7.86%) patients had nutcracker esophagus, 11 (12.35%) patients had scleroderma esophagus and 4 (4.49%) patients had achalasia. Two patients with nutcracker esophagus had hypertensive LES simultaneously. There was no patient who had an isolated LES abnormality (hypertensive LES, hypotensive LES and poorly relaxing LES). The additional viscous swallows did not change the diagnosis of achalasia, scleroderma esophagus and nutcracker esophagus. However, the manometric diagnoses of 8 among the 50 subjects who had normal manometry upon the liquid swallows were changed to IEM, and 4 among the 17 patients who had IEM upon the liquid swallows had their diagnosis changed to normal manometry upon the viscous swallows when we applied the same manometric criteria to the patients receiving viscous swallows. The final manometric diagnosis for each patient was based on the liquid swallows so as to remain consistent with the current tradition.
The bolus transit rate was high and the TBTT was short in the order of nutcracker esophagus, normal manometry and IEM, upon both liquid and viscous swallows (bP < 0.01). The liquid bolus moved faster than the viscous bolus (dP < 0.01, Table 1).
All patients with nutcracker esophagus had normal transit for the liquid and viscous swallows. For the subjects with normal manometry, 20% had abnormal liquid transit and 34% had abnormal viscous transit, and 46% of the patients with IEM had abnormal viscous transit. None of the patients with achalasia and scleroderma esophagus had normal transit for the liquid and viscous swallows.
In the analysis of individual swallows, the liquid bolus transit rates were 90% for the manometric normal swallows, 24% for the manometric ineffective swallows and 5% for the manometric simultaneous swallows. The viscous bolus transit rates were 83% for the manometric normal swallows, 9% for the manometric ineffective swallows, and 0% for the manometric simultaneous swallows (Table 2).
| Manometric evaluation | ||||||||
| Normal | Ineffective | Simultaneous | Total | |||||
| n | r% | n | r% | n | r% | n | ||
| MII evaluation | ||||||||
| Liquid | ||||||||
| Complete transit | n | 578 | 91.6 | 51 | 8.1 | 2 | 0.3 | 631 |
| C% | 89.6 | 23.7 | 4.9 | |||||
| Incomplete transit | n | 67 | 24.8 | 164 | 60.7 | 39 | 14.4 | 270 |
| C% | 10.4 | 76.3 | 95.1 | |||||
| Total | n | 645 | 71.6 | 215 | 23.9 | 41 | 4.6 | 901 |
| Viscous | ||||||||
| Complete transit | n | 423 | 96.1 | 17 | 3.9 | 0 | 0 | 440 |
| C% | 82.5 | 9.1 | 0 | |||||
| Incomplete transit | n | 90 | 29.6 | 170 | 55.9 | 44 | 14.5 | 304 |
| C% | 17.5 | 91.9 | 100 | |||||
| Total | n | 513 | 69.0 | 187 | 25.1 | 44 | 5.9 | 744 |
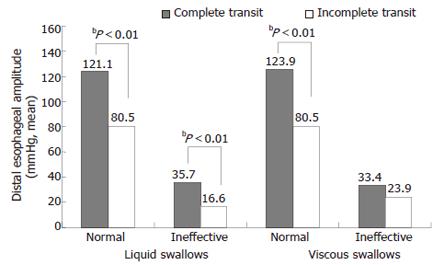
The distal esophageal amplitudes of the swallows with complete transit were significantly higher than those of the swallows with incomplete transit for both the liquid and viscous swallows, except for the manometric simultaneous swallows (P < 0.01, Figure 2).
The baseline impedance values for the patients with achalasia and scleroderma esophagus were lower than those of the subjects with normal manometry, IEM and nutcracker esophagus at all impedance measuring sites (P < 0.05, Table 3). There was no difference in the baseline impedance among all of the impedance-measuring sites for the patients with achalasia, but for the scleroderma patients, the impedance of the most proximal site was markedly higher than those of the other sites (P < 0.01).
| Impedance measuring site (distance from the LES) | ||||
| 20 cm (Ω) | 15 cm (Ω) | 10 cm (Ω) | 5 cm (Ω) | |
| Manometric diagnosis | ||||
| Liquid | ||||
| Normal | 1530 ± 549a | 1353 ± 464a | 1808 ± 608a | 2440 ± 778a |
| IEM | 1430 ± 572a | 994 ± 350a | 1318 ± 507a | 1875 ± 757a |
| Nutcracker | 1329 ± 539a | 1348 ± 550a | 1636 ± 712a | 2132 ± 1089a |
| Scleroderma | 1006 ± 503 | 448 ± 238 | 419 ± 194 | 402 ± 195 |
| Achalasia | 658 ± 478 | 456 ± 287 | 391 ± 140 | 660 ± 465 |
| Viscous | ||||
| Normal | 1415 ± 494a | 1317 ± 428a | 1589 ± 549a | 2518 ± 1452a |
| IEM | 1324 ± 476a | 1050 ± 289a | 1269 ± 465a | 1849 ± 742a |
| Nutcracker | 1447 ± 127a | 1302 ± 220a | 1642 ± 285a | 2206 ± 342a |
| Scleroderma | 787 ± 418 | 403 ± 160 | 378 ± 158 | 331 ± 145 |
| Achalasia | 388 ± 237 | 335 ± 150 | 323 ± 59 | 528 ± 343 |
Among the 37 subjects who had a 24-h pH study performed, 26 (70.27%) had normal results and the 11 (29.72%) subjects were diagnosed with GERD. The manometric diagnoses for the subject without pathologic gastroesophageal reflux were normal in 62%, IEM in 23% and nutcracker esophagus in 15%. The manometric diagnoses for the subjects with pathologic gastroesophageal reflux were normal in 73%, IEM in 18% and nutcracker esophagus in 9% cases. The distal esophageal amplitudes of the patients without pathologic reflux were higher than those of the subjects with pathologic gastroesophageal reflux (P < 0.05). The LES pressure, the pattern of esophageal contraction, bolus transit rate and the TBTT were not obviously different between them (Table 4).
| Swallow | Pathologicreflux (+) | Pathologicreflux (-) | P | |
| Manometry | ||||
| LES pressure (mmHg) | All | 21.9 ± 9.5 | 28.8 ± 9.5 | NS |
| Distal esophageal amplitude (mmHg) | Liquid | 113.6 ± 60.6 | 133.8 ± 78.5a | < 0.05 |
| Viscous | 113.2 ± 69.7 | 134.1 ± 79.1a | < 0.05 | |
| Impedance | ||||
| TBTT (s) | Liquid | 6.7 ± 1.5 | 6.6 ± 1.5 | NS |
| Viscous | 7.0 ± 1.0 | 7.1 ± 1.6 | NS | |
| Bolus transit rate (%) | Liquid | 91 ± 14 | 86 ± 16 | NS |
| Viscous | 73 ± 21 | 70 ± 30 | NS |
We evaluated the peristalsis and bolus transit in the subjects having abnormal and normal esophageal motility by using combined MII-EM. All the patients with achalasia and scleroderma esophagus, and some of the patients with IEM had abnormal bolus transit. Yet the patients with nutcracker esophagus had much better bolus transit than did the subjects having normal manometry.
The subjects having diverse esophageal motility abnormalities as well as the asymptomatic volunteers were included. Especially, the eleven patients with typical scleroderma esophagus were included. Most of the manometric ineffective swallows belonged to the scleroderma esophagus group and the IEM group. The bolus transit rate was markedly different between the IEM and scleroderma esophagus: 59% vs 9% on the liquid swallows and 61% vs 3% on the viscous swallows, respectively. Almost all the manometric simultaneous swallows belonged to the achalasia group. The bolus transit rate of the manometric simultaneous swallows was very low, less than 5%. We propose that the manometric ineffective or simultaneous swallows of patients with severe esophageal dysmotility and those of patients with the mild motility abnormality or normal motility should be classified and analyzed separately because their bolus transits were markedly different, as was seen by the results.
The esophageal body pressure, the LES pressure and the peristaltic movement can be considered as factors that have an influence on the bolus transit. This study showed that the major determinants of complete bolus transit were the esophageal body pressure and normal peristalsis. The bolus transit rate and the TBTT were better in the order of nutcracker esophagus, normal manometry and IEM. The higher the distal esophageal amplitude was, the better was the bolus transit. The contraction amplitudes of the swallows with complete transit were higher than those of the swallows with incomplete transit. So, it can be concluded that the esophageal body pressure is important to determine the complete bolus transit. Because there were no subjects with a poorly relaxing LES or hypotensive LES and only two patients with hypertensive LES in this study, we could not evaluate the effect of LES pressure on bolus transit. As the bolus transit rate and the TBTT of the two subjects with hypertensive LES who were classified as nutcracker esophagus were not different from those of the other subjects with nutcracker esophagus and normal LES pressure, we can guess that the high esophageal body amplitude overcomes the interference with bolus transit by the hypertensive LES. Almost all the swallows of the patients with achalasia and scleroderma esophagus had functional aperistalsis and incomplete bolus transit, so normal peristalsis is very important for bolus transit. If the subjects with diffuse esophageal spasm were included in this study, we could then know the role of isolated peristaltic abnormality on bolus transit. Our conclusion somewhat coincides with Tutuian et al[8] that the major determinant of bolus transit is the esophageal body pressure and any isolated LES pressure abnormality has a minor role. They suggested the new functional classification of esophageal motility abnormality: defects of bolus transit and isolated pressure abnormalities. The defects of bolus transit include achalasia, scleroderma esophagus, IEM and diffuse esophageal spasm. The isolated pressure defects include nutcracker esophagus, hypertensive LES, hypotensive LES and poor relaxation of the LES.
We suggest that the nutcracker esophagus should be classified as functionally normal as it had a better bolus transit than the manometrically normal esophagus. Up to now, esophageal hypercontraction abnormalities, such as hypertensive LES and nutcracker esophagus, have been the most controversial of the dysmotility patterns because it is not clear that esophageal hypercontraction has any physiological importance. Achalasia clearly showed low baseline impedance and failure of bolus transit. Retrograde esophageal contraction, intermittent reflux of the luminal contents and pathologic movement of luminal air during swallows seen in a previous study[9] were not clearly observed. Almost all the swallows of the patients with a scleroderma esophagus, in which the peristalsis was present faintly but very hypotentive, had incomplete transit like the achalasia patients. Because impedance testing has not yet been validated with radiographic studies in these patients, we should consider that impedance testing might overestimate incomplete transit. For example, the low pre-swallow impedance caused by the residual luminal content may influence the analysis of impedance of the following swallow.
There was no difference of baseline impedances among the patients with normal manometry, IEM and nutcracker esophagus. Compared to those, the baseline impedances of swallows with achalasia and scleroderma esophagus were significantly lower. Impedance correlates negatively with the cross sectional area of the esophagus and the conductivity of the luminal contents[10]. Low baseline impedance means the dilatation of lumen or the existence of luminal content. So, we could expect that the baseline impedance of the achalasia patients was significantly lower[11-13]. We had expected that impedance of the distal esophagus in the patients with achalasia would be higher than the impedance at the more proximal sites, but it was not. In the scleroderma esophagus, the impedance of the proximal esophagus was higher than that of the distal esophageal body. It means that the bolus transit of the upper esophagus and around the UES is preserved compared to the other sites. The low impedance of the distal esophagus adjacent to LES seemed to be associated with the decreased resistance of the gastroesophageal junction, which consists of smooth muscle[14]. The baseline impedance is considered to be the reflection of the dilatation of the esophageal body or the residual bolus produced by aperistalsis rather than it being a factor influencing bolus transit.
The bolus transit rate in the subjects with normal manometry was lower and had wider variation, as compared to previous studies. For example, one patient with normal manometry had a 20% transit rate for both liquid and viscous transit. Another patient with normal manometry had 100% liquid transit, but 0% viscous transit. The only 85% of the asymptomatic subjects with normal manometry had normal liquid transit and 78% had normal viscous transit. This showed that the precise evaluation of esophageal function was impossible via the indirect information from manometry only.
The viscous material may be a more sensitive material for the detection of minor motility abnormalities because generally, the viscous transit rate and transit time were worse than the liquid transit rate and transit time in the manometric normal and ineffective swallows. We should also consider the results of viscous swallows for the evaluation of esophageal dysmotility in that the manometric diagnoses of some patients with minor IEM or normal manometry were changed after applying the result of viscous swallows.
Some of the manometric ineffective and simultaneous swallows had complete transit, and there is the possibility of overestimation of the functional defect when using only the parameters of the manometry. The functional information from MII-EM will be useful for understanding the symptoms or pathophysiology of esophageal dysmotility which is minor or of unclear clinical significance such as nutcracker esophagus. However, the use of MII-EM is not always needed in daily clinical situation in that its result does not change the treatment option.
Impedance did not offer any additional information for the diagnosis of GERD. The differences of the distal esophageal amplitudes may be due to the fact that the group of patients without reflux included more number of patients with nutcracker esophagus. We had expected that the bolus transit of patients with pathologic gastroesophageal reflux would be worse because they had the abnormal acid clearance, but their parameters of impedance were not significantly different from those of the patients who were without gastroesophageal reflux.
In conclusion, the combined MII-EM provides delicate and functional informations about the bolus transit of normal subjects and the patients with minor esophageal dysmotility as well as severe esophageal dysmotility. The major factors to determine the complete bolus transit are the esophageal body pressure and normal peristalsis. The validation of measuring impedance along with performing radiographic study will be needed for the patients with esophageal motility abnormality, and its clinical and prognostic value should be clarified by an outcome study[15].
S- Editor Wang GP L- Editor Kumar M E- Editor Bi L
| 1. | Richter JE, Blackwell JN, Wu WC, Johns DN, Cowan RJ, Castell DO. Relationship of radionuclide liquid bolus transport and esophageal manometry. J Lab Clin Med. 1987;109:217-224. [PubMed] |
| 2. | Nguyen HN, Domingues GR, Winograd R, Koppitz P, Lammert F, Silny J, Matern S. Impedance characteristics of normal oesophageal motor function. Eur J Gastroenterol Hepatol. 2003;15:773-780. [PubMed] |
| 3. | Fass J, Silny J, Braun J, Heindrichs U, Dreuw B, Schumpelick V, Rau G. Measuring esophageal motility with a new intraluminal impedance device. First clinical results in reflux patients. Scand J Gastroenterol. 1994;29:693-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 88] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Nguyen HN, Silny J, Albers D, Roeb E, Gartung C, Rau G, Matern S. Dynamics of esophageal bolus transport in healthy subjects studied using multiple intraluminal impedancometry. Am J Physiol. 1997;273:G958-G964. [PubMed] |
| 5. | Imam H, Shay S, Ali A, Baker M. Bolus transit patterns in healthy subjects: a study using simultaneous impedance monitoring, videoesophagram, and esophageal manometry. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1000-G1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 72] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 6. | Simrén M, Silny J, Holloway R, Tack J, Janssens J, Sifrim D. Relevance of ineffective oesophageal motility during oesophageal acid clearance. Gut. 2003;52:784-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 185] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 7. | Spechler SJ, Castell DO. Classification of oesophageal motility abnormalities. Gut. 2001;49:145-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 499] [Cited by in RCA: 466] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 8. | Tutuian R, Vela MF, Shay SS, Castell DO. Multichannel intraluminal impedance in esophageal function testing and gastroesophageal reflux monitoring. J Clin Gastroenterol. 2003;37:206-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 100] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 9. | Nguyen HN, Domingues GR, Winograd R, Lammert F, Silny J, Matern S. Impedance characteristics of esophageal motor function in achalasia. Dis Esophagus. 2004;17:44-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Nguyen HN, Silny J, Matern S. Multiple intraluminal electrical impedancometry for recording of upper gastrointestinal motility: current results and further implications. Am J Gastroenterol. 1999;94:306-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 60] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Ott DJ, Richter JE, Chen YM, Wu WC, Gelfand DW, Castell DO. Esophageal radiography and manometry: correlation in 172 patients with dysphagia. AJR Am J Roentgenol. 1987;149:307-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 77] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Margulis AR, Koehler RE. Radiologic diagnosis of disordered esophageal motility: a unified physiologic approach. Radiol Clin North Am. 1976;14:429-439. [PubMed] |
| 13. | Stewart ET. Radiographic evaluation of the esophagus and its motor disorders. Med Clin North Am. 1981;65:1173-1194. [PubMed] |
| 14. | Mearin F, Fonollosa V, Vilardell M, Malagelada JR. Mechanical properties of the gastro-esophageal junction in health, achalasia, and scleroderma. Scand J Gastroenterol. 2000;35:705-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Shay S. Esophageal impedance monitoring: the ups and downs of a new test. Am J Gastroenterol. 2004;99:1020-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |