INTRODUCTION
Propylthiouracil (PTU)-related severe hepatitis remains a relatively rare occurrence[1,2] even if a transient increase in liver function tests is observed following the initiation of treatment in 15% to 28% of cases[2-4]. Before 1998, only 25 cases of PTU-induced severe liver injury were reported[5] and 83 cases with 28 deaths were included in a recent review of literature[6]. We here report two additional cases of PTU-induced severe liver injury, one was complicated by liver failure with hepatic encephalopathy. The late occurrence of liver toxicity and the underestimation of liver damage together with delayed drug withdrawal likely play an important role in their severity.
CASE REPORT
Case 1
A 35-year old woman was referred to our unit on February 4, 2004 for a two-month history of jaundice and pruritus. She was treated for hyperthyroidism with thiamazol, thiamazol and levothyroxin in 1997-1999. In early July 2003 three months following an uneventful delivery, she presented with features of relapsing hyperthyroidism, with her thyroid-stimulating hormone (TSH) unmeasurable (normal range: 0.2-3.5 microU/mL), T4 > 6 ng/dL (normal range: 0.8-2 ng/mL) and a strong positivity of antithyroid antibodies. She was treated with PTU (150 mg per day during the first 2 mo followed by 100 mg per day). In November 2003, she experienced upper abdominal discomfort, nausea and vomiting together with a weight-loss of 4 Kg. Thyroid testing performed on December 16 showed: TSH: 0.04, T4: O.95, free T3: 9.71. Liver biochemistry showed an increase in aspartate aminotransferase (AST) at 495 IU/L (normal value < 33) and ALT at 1575 IU/mL (normal value < 63). The total serum bilirubin level was 10 mg/dL. Despite this worsening in liver function test (LFT), therapy with PTU was however continued till its delayed withdrawal on January 21, 2004. She was referred to our unit on February 4, 2004 due to jaundice and fatigue together with the persistence of upper gastrointestinal (GI) symptoms.
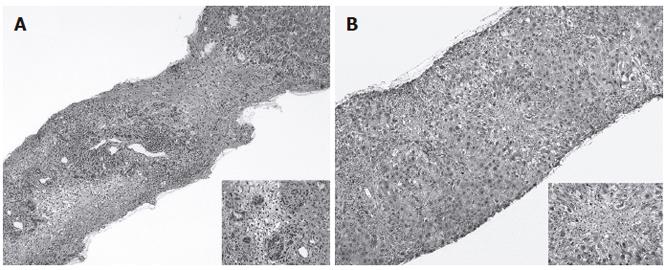
She was deeply icteric with a hepatomegaly 4 cm under the right costal margin. Liver biochemistry showed alanine aminotransferase (ALT): 458 IU/L, AST: 307 IU/L, alkaline phosphatase (Alk. Phos): 139 IU/L (normal value < 94 IU/L), lactate dehydrogenase (LDH): 239 IU/L (normal value < 192 IU/L), and total s. bilirubin at 22.2 mg/dL. Serology for hepatitis A, B, C, CMV, EBV and herpes was negative. There was a slight positivity of pANCA and cANCA (1/160). At the time of admission, TSH was < 0.01, T4: 3.5 and anti-TPO antibodies: 665 (normal value < 100 U/mL). Upper abdominal ultrasound was unremarkable. Magnetic resonance imaging showed the presence of bands of necrosis scattered throughout the liver parenchyma. At transjugular hepatic vein catheterisation, the hepatic venous pressure was 9 mmHg suggestive of post-sinusoidal portal hypertension. A transvenous liver biopsy specimen showed replacement of the normal architecture by areas of multilobular necrosis infiltrated by mononuclear inflammatory cells and regenerating bile ducts (Figure 1A). The few preserved portal structures were enlarged and infiltrated, also showing interface hepatitis. Shortly after admission the patient exhibited features of hepatic encephalopathy with confusion, tremor and agitation which lasted for 8 d. EEG showed features compatible with severe liver encephalopathy (grade 3 of Child’s EEG scoring system). The biochemical condition then slowly improved, hyperthyroidism was treated with thiamazol (20 mg per day followed by 10 mg per day). The patient was discharged after 13 d, while her clinical and biochemical resolution occurred within 4 mo.
Figure 1 Liver histology of case 1 showing a picture of panlobular necrosis infiltrated with dense inflammatory infiltrates and ductular proliferation at higher magnification (close-up) (A), liver histology of case 2 showing enlarged and infiltrated portal tracts with mild interface hepatitis with prominent lobular necrosis in the centrilobular regions (close-up) (B).
Case 2
A 43-year old female was referred to our unit on March 6, 2004 for a history of 10-d jaundice, fever and upper GI symptoms. She had no previous medical history except for hyperthyroidism diagnosed in August 2003. The condition was first treated with thiamazol which was however rapidly withdrawn due to the occurrence of skin rash. A treatment with PTU (100 mg per day) was initiated in early October 2003, levothyroxin (75 mg/d) was added shortly therafter. At clinical examination upon referral, the patient was icteric with no sign of hyperthyroidism. The liver was felt 3 cm under the right costal margin. Liver biochemistry showed: total s. bilirubin: 10.9 mg/dL, AST: 2310 IU/L, ALT: 5040 IU/L. Serology was negative for hepatitis A and B as well as for antinuclear and smooth muscle antibodies. CMV-IgM as well as EBV-IgM antibodies were slightly positive. TSH was 4.09 micro U/mL, T4: 1.2, free T3: 1.9. Anti-TPO and anti-TG antibodies were both negative. Upper abdominal ultrasound showed a normal size hyperechogenic liver parenchyma. A transcutaneous liver biopsy was obtained which showed widely enlarged portal tracts infiltrated with lymphocytes and neutrophils together with ductular proliferation. Interface hepatitis was clearly visible and there were prominent areas of centrilobular necrosis infiltrated with lymphocytes in the lobules (Figure 1B). Acidophilic bodies were scattered into the parenchyma. Immunostaining for both EBV and CMV was negative. Following drug withdrawal the clinical condition of the patient progressively improved together with the normalisation of liver function tests which occurred after 8 wk of follow-up. Thyroid testing remained in the normal range.
DISCUSSION
We reported two cases of severe PTU-induced hepatitis, one appearing 5 mo after the initiation of therapy and the other one after 6 mo. Liver histology of the first case showed multilobular necrosis, the disease ran a subfulminant course complicated by hepatic encephalopathy and liver failure. In the second case characterised by morphological features of centrilobular necrosis, the clinical and biological evolution was less severe, but ran a prolonged disabling course. The diagnosis of PTU-induced hepatitis was based on the occurrence of liver damage within the first few months of therapy, the absence of previous history and/or other causes of liver disease and the recovery following its discontinuation.
Changes in liver function tests occurring in hyperthyroidism have been known for a long time. Abnormal liver function tests can be observed in 15%-76% of the cases. The pathophysiology of these changes remains unknown, likely involving multifactorial factors including liver congestion, increased liver oxygen consumption, reduced bile acid synthesis, and/or glycuronyl-transferase inhibition[3,7]. In this setting liver histology only shows non-specific changes[8].
On the contrary, PTU-induced acute hepatitis occurs in 0.1%-1.2% of treated patients[4,9,10]. This rate is much higher than that observed in the large context of liver immuno-allergic toxicity (1/10 000 to 1/100 000 or even to 1/1 000 000 for the majority of drugs)[11], and much lower than that predicted by an asymptomatic increase in ALT which is observed in about 14%-28% of cases during the first week of therapy[2,10]. In the presence of acute hepatitis the mortality may be as high as 25%[12] and so far only 28 lethal cases have been reported in the English literature[6], with two additional cases successfully transplanted[12,13]. As in our case reports, histological features liver necrosis of variable severity with or without cholestasis[10], suggesting the role of an immuno-allergic type of toxicity irrespective of the doses of drug used[5].
In our cases, the delay between the initiation of therapy and the occurrence of symptoms of hepatitis was 5 and 6 mo, respectively. This is in agreement with previous reports[3,14,15]. The majority of cases are however observed during the first 6 mo of therapy[1].The positivity of antineutrophil cytoplasmic antibody (ANCA) testing is not surprising since PTU is a potential causative agent of ANCA positive vasculitis[16].
In our first observation thiamazol was substituted with PTU without side effect as in another reported case in which carbimazole was used as a substitute to PTU[17]. Convincing descriptions of thiamazol and PTU hepatic cross-toxicity are indeed lacking, even if cross-sensitivity may occur[18] and if the rate of untoward cross-reaction between carbimazole and PTU can reach 15%[18].
In conclusion, this report reinforces the warning against the potential for PTU to induce severe life-threatening hepatitis. The incidence of transient increase in liver function tests at the initiation of treatment reaches about 28%, while the incidence of severe toxicity is about 1%, a risk being much higher than that observed in the majority of compounds considered as potential hepatotoxins. Due to this high incidence of liver toxicity and the rather short delay between the initiation of treatment and the occurrence of hepatitis as well as its relatively slow “subacute” progression, ALT monitoring may be cost-effective in reducing the severity of the liver side effect. The duration of optimal biochemical follow-up remains to be further evaluated in large prospective trials but should be about six months, during which the highest rate of PTU-induced hepatitis is observed.