Published online Oct 14, 2006. doi: 10.3748/wjg.v12.i38.6178
Revised: June 28, 2006
Accepted: July 7, 2006
Published online: October 14, 2006
AIM: To investigate the effect of polaprezinc on cellular damage induced by hydrogen peroxide (H2O2) in human colon CaCo2 cells.
METHODS: CaCo2 cells were treated with polaprezinc (10-100 μmol/L) for 6 h. After polaprezinc treatment, the cells were incubated with H2O2 (20 μmol/L) for 1 h. Cell viability was measured by MTT assay. Western blot analysis for heat shock protein (HSP) 27 and HSP72 in the cells was performed. Moreover, cells were pretreated with quercetin (200 μmol/L), an inhibitor of HSP synthesis, 2 h before polaprezinc treatment, and cell viability and the expression of HSP27 and 72 were assessed in these cells.
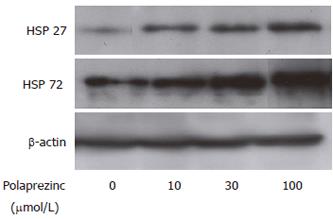
RESULTS: Polaprezinc significantly protected CaCo2 cells from cell damage induced by H2O2, and up-regulated the expressions of HSP27 and HSP72 in the cells (10, 30 and 100 μmol/L of polaprezinc; 35.0% ± 7.7%, 58.3% ± 14.6% and 64.2% ± 8.2%, respectively. P < 0.01 versus polaprezinc-nontreated cells; 6.0% ± 4.4%). Quercetin inhibited the up-regulation of HSP27 and HSP72 by polaprezinc and diminished the protective effect of polaprezinc against H2O2-caused injury in the cells.
CONCLUSION: Polaprezinc is a useful therapeutic agent for treatment of colitis and its effects depend on the function of cytoprotective HSP in colon.
- Citation: Ohkawara T, Nishihira J, Nagashima R, Takeda H, Asaka M. Polaprezinc protects human colon cells from oxidative injury induced by hydrogen peroxide: Relevant to cytoprotective heat shock proteins. World J Gastroenterol 2006; 12(38): 6178-6181
- URL: https://www.wjgnet.com/1007-9327/full/v12/i38/6178.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i38.6178
Polaprezinc [N-(3-aminopropionyl)-L-histidinato zinc], an antiulcer drug, is a chelate compound consisting of zinc ion, L-carnosine, dipeptide of β-alanine, and L-histidine and has an antioxidant effect and anti-H pylori activity[1-4]. It has been reported that administration of polaprezinc prevents gastric mucosa from tissue injury in experimental models[5-9]. Additionally, recent works indicate that polaprezinc has a therapeutic effect in two models of experimental colitis[10,11]. On the other hand, some studies have shown that polaprezinc up-regulates the expression of heat shock protein (HSP) in stomach and colon[11,12].
HSP, a highly conserved and ubiquitous protein, is up-regulated to protect against various physiological stress conditions such as infection and ishcemia[13]. Some HSPs are now accepted to be key anti-inflammatory molecules and play an important role in the protection against physiologic and environmental stressors[14]. Overexpression of these HSPs are thought to prevent apoptosis by regulating intracellular intermediates intimately involved in apoptotic signaling. In intestine, up-regulation of these HSPs by chemicals or non-lethal thermal stress has been shown to protect intestinal epithelial cells and colon tissues against injurious stimulants in vitro and in vivo[15-20]. In particular, HSP27 and HSP72 protect cells and tissues from chemical, infectious and ischemic injury[13]. On the other hand, our previous study demonstrated that polaprezinc up-regulates the expression of HSP27 and HSP72 in the mouse colon[11], but there are no investigations of the effect of polaprezinc on HSP expression in intestinal epithelial cells in vitro. Thus, we herein assessed the effect of polaprezinc on cell damage induced by oxidative stress, which often occurs in intestine and injured colonic epithelial cells in vitro. Moreover, we investigated whether the protective effect of polaprezinc depends on the function of HSPs in human colon cells.
Polaprezinc was a gift from Zeria Pharmaceuticals Co. (Saitama, Japan). Antibodies against HSP25 and HSP72 were obtained from Stressgen (Victoria, BC, Canada). Nitrocellulose membrane filters were from Millipore (Bedford, MA). An ECL Western blotting detection system kit was from Amersham Bioscience (Piscataway, NJ, USA). Horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG antibody and a Micro BCA protein assay kit were from Piece (Rockford, IL, USA). Quercetin was from Wako Pure Chemical Industries (Osaka, Japan). A CellTiter 96 aqueous one solution cell proliferation assay kit was from Promega (Madison, WI, USA) and an anti-actin antibody was from Sigma-Aldrich Co. (Temecula CA, USA). All other chemicals were of reagent grade.
CaCo2 cells (between passages 8 and 14) were grown in high glucose Dulbeco’s Vogt modified Eagle’s media (DMEM, Sigma, St Louis, MO, USA) supplemented with 100 mL/L fetal calf serum. Cells were incubated at 37°C in 50 mL/L CO2 and 90% humidity. Each experiment was performed with an 80%-90% confluent monolayer. Polaprezinc was diluted in DMEM and added to the cultured cells at a final concentration of 10-100 μmol/L for 6 h.
To examine the protective effect of polaprezinc on CaCo2 cells against oxidant-induced injury, we assessed cell viability by MTT assay as described previously[20]. Briefly, cells were grown to an approximate cell concentration of 104 cells/well in 96-well plates. Cells in each well were incubated at 37°C with 150 μL of DMEM and 50 μL of CellTiter 96 aqueous, one solution cell proliferation reagent. Cell viability was determined by the generation of a formazan dye from the substrate. Absorbance (A) at 490 nm was measured with a spectrometer 0 and 90 min after addition of CellTiter 96 aqueous, one solution cell proliferation reagent. Difference in A between 0 and 90 min after incubation was calculated for evaluation of cell viability. The assay was run on cells without treatment as a negative control. The results were compared to those in a negative control and expressed as percentage of cell viability. All experiments were repeated more than three times to confirm reproducibility.
Cells were collected with 1 g/L trypsin and homogenated with a Polytron homogenizer (Kinematica, Lucerne, Switzerland). Equal amounts of homogenates were dissolved in 20 μL of Tris-HCL, 50 mmol/L (pH 6.8), containing 10 g/L 2-mercaptoethanol, 20 g/L SDS, 200 mL/L glycerol and 0.4 g/L bromophenol blue. The samples were heated at 100°C for 5 min, then subjected to SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred electrophoretically onto a nitrocellulose membrane. The membranes were blocked with 10 mL/L nonfat dry milk in PBS, probed with HSP 27 and 72 Ab, and reacted with goat anti-rabbit IgG Ab coupled with horseradish peroxidase (HRP). The resultant complexes were processed for the ECL detection system according to the manufacturer’s protocol. Protein concentration in the homogenate was quantified using a Micro BCA protein assay reagent kit (Pierce, Rockford, IL, USA).
Quercetin is known to strongly inhibit the HSP synthesis[15,20,21]. To investigate the effect of quercetin, cells were pretreated with quercetin at a final concentration of 200 μmol/L for 2 h before polaprezinc treatment. Cell viability and expression of HSP27 and HSP72 were assessed in the cells treated with quercetin.
Data obtained by MTT assay were presented as mean ± SD and statistically analyzed using an analysis of variance (ANOVA), followed by Turkey’s comparison test (Stat View, SAS Institute, Cary, NC). P < 0.05 was considered statistically significant.
We assessed the effect of polaprezinc on oxidative injury in the CaCo2 cells. Morphological alteration and growth inhibition were not observed in the CaCo2 cells after exposure to polaprezinc (data not shown). To evaluate the effect of polaprezinc on oxidative stress, cell viability in CaCo2 cells treated with NH2Cl was analyzed by MTT assay. MTT assay showed that, the difference in A at 490 nm, as a parameter of cell viability, was significantly decreased in the cells treated with hydrogen peroxide at a final concentration of 20 mol/L (6.0% ± 4.4%). In contrast, we found that 10, 30 and 100 μmol/L of polaprezinc (35.0% ± 7.7%, 58.3% ± 14.6% and 64.2% ± 8.2%, respectively) significantly improved viability in the cells at 6 h after polaprezinc treatment compared with the cells without polaprezinc treatment (P < 0.01).
For assessment of the HSP expression in the CaCo2 cells treated with polaprezinc, Western blot analysis for HSPs in the cells was carried out. It was found that HSP27 and HSP72 were constitutively expressed in the CaCo2 cells without treatment. The expressions of HSP25 and HSP72 was greatly up-regulated in the CaCo2 cells treated with 10, 30 and 100 μmol/L of polaprezinc for 6 h compared with those in the non-treated CaCo2 cells (Figure 1).
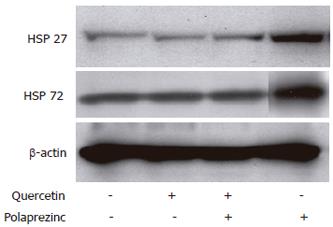
To clarify the effect of HSPs induced by polaprezinc on cell injury, we investigated the effect of quercetin, an inhibitor of HSP synthesis, and polaprezinc on Caco2 cells injured by H2O2. Pretreatment with 200 μmol/L of quercetin for 2 h considerably reduced the cell viability in the CaCo2 cells treated with 30 μmol/L of polaprezinc compared with the cells without quercetin treatment when cells were exposed to H2O2 (P < 0.01; 12.0% ± 0.3% and 53.6% ± 1.9%, respectively), whereas the cell viability in the quercetin-treated CaCo2 cells without H2O2 was minimal compared with non-treated cells (94.3% ± 0.1% and 98.8 % ± 0.1%, respectively). In HSP expression, 200 μmol/L of quercetin completely inhibited the up-regulation of HSP27 and HSP72 by polaprezinc in the CaCo2 cells (Figure 2). On the other hand, quercetin did not alter the baseline level of HSP27 and HSP72 in the cells without polaprezinc treatment (Figure 2).
In this study, we demonstrated that polaprezinc improved cell viability of CaCo2 cells injured by oxidative chemicals. Moreover, polaprezinc remarkably up-regulated the expression of HSP27 and HSP72, which play an important role in protecting the cells from stresses. Additionally, quercetin, an inhibitor of HSP synthesis diminished the protective effect of polaprezinc on cell injury by H2O2 and the up-regulation of HSP27 and HSP72 in CaCo2 cells. These results are consistent with our previous observation that polaprezinc can up-regulate the expressions of HSP27 and HSP70 in murine experimental colitis[11]. Recent studies have demonstrated that the expression of HSP27 and HSP70 is up-regulated by mild physiological stress and irritants[13,14]. Furthermore, HSPs have been suggested to be key anti-inflammatory molecules and play a critical role in protective mechanism against severe physiologic and environmental stressors[13,14]. High expression levels of HSPs are thought to prevent apoptosis by regulating intracellular intermediates intimately involved in apoptotic signaling.
In intestine, induction of HSPs by chemicals or non-lethal thermal stress has been shown to protect intestinal cells and colon tissues against injury and damage[15-19]. Musch et al[15] reported that induction of HSP72 by hyperthermia protects rat intestinal epithelial cells from oxidative injury. Hyperthermia rapidly and reproducibly induces HSPs in intestinal epithelial cells. Otani et al[16] demonstrated that preinduction of HSPs by non-lethal hyperthermia protects rats from colitis induced by acetic acid. Hyperthermia elevates the expression of HSPs, including HSP72, and remarkably reduces the severity of acetic acid-induced colitis in the colons of rats, suggesting that HSPs are important for the protection against colon tissue damage such as colitis. Although numerous studies have clarified the protecting effect of polaprezinc, there are a few investigations on the up-regulation of HSP by polaprezinc. Very recently, it has been reported that polaprezinc up-regulates the expression of HSP72 in cultured rat gastric mucosal cells (RGM1) and rat gastric mucosa[12]. In colon, our previous study demonstrated that polaprezinc enhances the up-regulation of HSP72 in mouse colon during colitis[11]. However, these studies have not fully clarified the role of HSP induced by polaprezinc. In this study, we demonstrated that quercetin, an inhibitor of HSP synthesis, diminished the protective effect of polaprezinc on cell damage induced by H2O2 and up-regulated HSP, suggesting that polaprezinc-induced HSP is essential for protection against oxidative injury in colon cells.
Besides HSP70, HSP27, a member of the small HSP family, have also been found to play an important role in protection of cells against stresses[13]. Ropeleski et al[18] demonstrated that IL-11-induced HSP27 has a protectiefve effect against oxidative stress induced by monochrolamine in cultured rat intestinal epithelial cells, as HSP27 plays a potential role in cytoprotection in intestine. Consistent with these findings and our previous findings, the expression of HSP27 is markedly up-regulated in the CaCo2 cells treated with polaprezinc. Howevere, quercetin did not up-regulate the expression of HSP27 in CaCo2 cells treated with polaprezinc in our study. These findings provide the idea that the protective effect of polaprezinc depends not only on the function of HSP72 but also on HSP27 in colon cells.
The mechanism by which polaprezinc up-regulates the expression of HSPs has not been precisely elucidated. Interestingly, it has been reported that zinc or L-carnocine cannot up-regulate the expression of HSPs in gastric epithelial cells and rat gastric mucosa[12]. Our previous study indicates that zinc supplement does not induce HSPs in mouse colon[11]. Although we did not show the effects of zinc or L-carnocine on induction of HSPs in colon cells, polaprezinc may be a powerful inducer of HSPs compared with zinc or L-carnocine. The reason why polaprezinc but not zinc or L-carnocine markedly up-regulates the expression of HSPs is unclear, thus, further study is needed to clarify this result.
In conclusion, polaprezinc protects colon cells from oxidative injury induced by hydrogen peroxide, and enhances the expression of HSP27 and HSP72 in the CaCo2 cells. The up-regulation of cytoprotective HSPs by polaprezinc is potentially therapeutic for intestinal injuries such as colitis.
S- Editor Pan BR L- Editor Wang XL E- Editor Bi L
| 1. | Yoshikawa T, Naito Y, Tanigawa T, Yoneta T, Kondo M. The antioxidant properties of a novel zinc-carnosine chelate compound, N-(3-aminopropionyl)-L-histidinato zinc. Biochim Biophys Acta. 1991;1115:15-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 82] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 2. | Yoshikawa T, Naito Y, Tanigawa T, Yoneta T, Yasuda M, Ueda S, Oyamada H, Kondo M. Effect of zinc-carnosine chelate compound (Z-103), a novel antioxidant, on acute gastric mucosal injury induced by ischemia-reperfusion in rats. Free Radic Res Commun. 1991;14:289-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 57] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | Handa O, Yoshida N, Tanaka Y, Ueda M, Ishikawa T, Takagi T, Matsumoto N, Naito Y, Yoshikawa T. Inhibitory effect of polaprezinc on the inflammatory response to Helicobacter pylori. Can J Gastroenterol. 2002;16:785-789. [PubMed] |
| 4. | Naito Y, Yoshikawa T, Yagi N, Matsuyama K, Yoshida N, Seto K, Yoneta T. Effects of polaprezinc on lipid peroxidation, neutrophil accumulation, and TNF-alpha expression in rats with aspirin-induced gastric mucosal injury. Dig Dis Sci. 2001;46:845-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Ishihara R, Iishi H, Sakai N, Yano H, Uedo N, Narahara H, Iseki K, Mikuni T, Ishiguro S, Tatsuta M. Polaprezinc attenuates Helicobacter pylori-associated gastritis in Mongolian gerbils. Helicobacter. 2002;7:384-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Korolkiewicz RP, Fujita A, Seto K, Suzuki K, Takeuchi K. Polaprezinc exerts a salutary effect on impaired healing of acute gastric lesions in diabetic rats. Dig Dis Sci. 2000;45:1200-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Ueki S, Seiki M, Yoneta T, Omata T, Hori Y, Ishikawa M, Tagashira E. Effect of Z-103 on compound 48/80-induced gastric lesions in rats. Scand J Gastroenterol Suppl. 1989;162:202-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Sugawara N, Katakura M, Sugawara C. Preventive effect of zinc compounds, polaprezinc and zinc acetate against the onset of hepatitis in Long-Evans Cinnamon rat. Res Commun Mol Pathol Pharmacol. 1999;103:167-176. [PubMed] |
| 9. | Katayama S, Nishizawa K, Hirano M, Yamamura S, Momose Y. Effect of polaprezinc on healing of acetic acid-induced stomatitis in hamsters. J Pharm Pharm Sci. 2000;3:114-117. [PubMed] |
| 10. | Yoshikawa T, Yamaguchi T, Yoshida N, Yamamoto H, Kitazumi S, Takahashi S, Naito Y, Kondo M. Effect of Z-103 on TNB-induced colitis in rats. Digestion. 1997;58:464-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Ohkawara T, Takeda H, Kato K, Miyashita K, Kato M, Iwanaga T, Asaka M. Polaprezinc (N-(3-aminopropionyl)-L-histidinato zinc) ameliorates dextran sulfate sodium-induced colitis in mice. Scand J Gastroenterol. 2005;40:1321-1327. [RCA] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Odashima M, Otaka M, Jin M, Konishi N, Sato T, Kato S, Matsuhashi T, Nakamura C, Watanabe S. Induction of a 72-kDa heat-shock protein in cultured rat gastric mucosal cells and rat gastric mucosa by zinc L-carnosine. Dig Dis Sci. 2002;47:2799-2804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Binder RJ, Vatner R, Srivastava P. The heat-shock protein receptors: some answers and more questions. Tissue Antigens. 2004;64:442-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 171] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 14. | Pockley AG. Heat shock proteins as regulators of the immune response. Lancet. 2003;362:469-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 528] [Cited by in RCA: 500] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 15. | Musch MW, Ciancio MJ, Sarge K, Chang EB. Induction of heat shock protein 70 protects intestinal epithelial IEC-18 cells from oxidant and thermal injury. Am J Physiol. 1996;270:C429-436. |
| 16. | Otani S, Otaka M, Jin M, Okuyama A, Itoh S, Iwabuchi A, Sasahara H, Itoh H, Tashima Y, Masamune O. Effect of preinduction of heat shock proteins on acetic acid-induced colitis in rats. Dig Dis Sci. 1997;42:833-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Ren H, Musch MW, Kojima K, Boone D, Ma A, Chang EB. Short-chain fatty acids induce intestinal epithelial heat shock protein 25 expression in rats and IEC 18 cells. Gastroenterology. 2001;121:631-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 85] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 18. | Ropeleski MJ, Tang J, Walsh-Reitz MM, Musch MW, Chang EB. Interleukin-11-induced heat shock protein 25 confers intestinal epithelial-specific cytoprotection from oxidant stress. Gastroenterology. 2003;124:1358-1368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 77] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | Kojima K, Musch MW, Ren H, Boone DL, Hendrickson BA, Ma A, Chang EB. Enteric flora and lymphocyte-derived cytokines determine expression of heat shock proteins in mouse colonic epithelial cells. Gastroenterology. 2003;124:1395-1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 86] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 20. | Ohkawara T, Takeda H, Nishiwaki M, Nishihira J, Asaka M. Protective effects of heat shock protein 70 induced by geranylgeranylacetone on oxidative injury in rat intestinal epithelial cells. Scand J Gastroenterol. 2006;41:312-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Hosokawa N, Hirayoshi K, Kudo H, Takechi H, Aoike A, Kawai K, Nagata K. Inhibition of the activation of heat shock factor in vivo and in vitro by flavonoids. Mol Cell Biol. 1992;12:3490-3498. [PubMed] |