Published online Oct 14, 2006. doi: 10.3748/wjg.v12.i38.6133
Revised: June 8, 2006
Accepted: June 14, 2006
Published online: October 14, 2006
AIM: To analyze the level of apoptosis in different mucosal compartments and the differential expression of Fas/Fas-ligand and perforin in H pylori-associated gastric ulcer.
METHODS: Antral specimens from patients with H pylori-related active gastric ulcer (GU), H pylori-related gastritis, and non-infected controls were analysed for densities and distribution of apoptotic cells determined by the TdT-mediated dUDP-biotin nick-end-labelling method. GU patients were submitted to eradication therapy with follow-up biopsy after 60 d. Fas, FasL, and perforin-expressing cells were assessed by immunoperoxidase, and with anti-CD3, anti-CD20 and anti-CD68 by double immunofluorescence and confocal microscopy. Quantitative analysis was performed using a computer-assisted image analyser.
RESULTS: H pylori-infected antrum showed greater surface epithelial apoptosis which decreased after eradication therapy. In the lamina propria, higher rates of mononuclear cell apoptosis were observed in H pylori-gastritis. Co-expression of Fas with T-cell and macrophage markers was reduced in GU. FasL- and perforin-expressing cells were increased in H pylori-infection and correlated with epithelial apoptosis. Perforin-expressing cells were also increased in GU compared with H pylori-gastritis.
CONCLUSION: Epithelial apoptosis is increased in H pylori-infection and correlates to FasL- and perforin-expression by T cells. Expression of perforin is correlated with the tissue damage, and may represent the enhancement of a distinct cytotoxic pathway in GU. Increased expression of FasL not paralleled by Fas on T-cells and macrophages may indicate a reduced susceptibility to the Fas/FasL-mediated apoptosis of lymphoid cells in H pylori-infection.
-
Citation: Souza HS, Neves MS, Elia CC, Tortori CJ, Dines I, Martinusso CA, Madi K, Andrade L, Castelo-Branco MT. Distinct patterns of mucosal apoptosis in
H pylori -associated gastric ulcer are associated with altered FasL and perforin cytotoxic pathways. World J Gastroenterol 2006; 12(38): 6133-6141 - URL: https://www.wjgnet.com/1007-9327/full/v12/i38/6133.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i38.6133
Infection with H pylori induces chronic gastritis in virtually all infected hosts and it has been implicated as the major etiologic factor in peptic ulcer disease, chronic atrophic gastritis[1,2], gastric carcinoma, and gastric lymphoma[3,4]. An important feature of H pylori infection relies on the fact that the gastric mucosal immune response is not capable of eliminating the organism, and the infection persists indefinitely[5]. The local inflammatory process is constituted by immunocompetent cells, including a large number of T cells in the lamina propria and an increased expression of T-helper type 1 (Th1) proinflammatory cytokines[6,7].
H pylori has been reported to increase apoptosis of gastric epithelial cells which has been associated with gastric atrophy and also peptic ulcer[8]. Although the mechanisms by which the infection with H pylori may result in abnormal apoptosis are still unclear, there is some evidence indicating a major role for the host immune response. Results from an in vitro study demonstrated that interferon gamma (IFN-gamma) increases the attachment of the bacteria as well as the induction of apoptosis in gastric epithelial cell lines exposed to H pylori[9]. In another in vitro study, proinflammatory stimuli such as tumor necrosis factor alpha, IFN-gamma, and a receptor-activating CD95/APO-1/Fas antibody, were shown to potentiate H pylori-induced apoptosis both directly and indirectly through sensitization of epithelial cells[10].
Alterations in apoptosis have been associated with the pathogenesis of diseases such as cancer, viral infections, autoimmunity[11], and inflammatory bowel disease[12,13]. The increased apoptosis of epithelial cells triggered by H pylori has been related to the enhancement of the Fas/Fas ligand signaling pathway in the gastric mucosa[14,15]. Results from in vitro studies have demonstrated a possible role for the Fas-mediated cytotoxicity of activated gastric T-cell lines in the apoptosis of gastric epithelial cells during the infection with H pylori[16,17].
Apoptosis mediated by cytotoxic T lymphocytes (CTLs) is thought to constitute an essential mechanism of recognition of auto-reactive cells and of target-cell destruction. Although increased gastric epithelial apoptosis has been associated with the expansion of mucosal T-helper type 1 cells, the role of T-cell mediated cytotoxicity in that process is still unclear. Therefore, this study was designed to evaluate the rate of apoptosis in different mucosal compartments of H pylori-associated gastric ulcer disease. In addition, distinct pathways of cytotoxicity were assessed through the differential expression of Fas and Fas ligand, and of perforin in the gastric mucosa of patients in different clinical conditions including after eradication therapy.
Fifteen consecutive patients with H pylori-positive active gastric peptic ulcers, and 14 H pylori-positive and 14 H pylori-negative patients both with endoscopically normal gastroduodenal mucosa were enrolled in this study. None of the patients studied had received any antibiotics, nonsteroidal anti-inflammatory drugs, bismuth compounds, H2-receptor antagonists, proton pump inhibitors, anticancer drugs, or corticosteroids at least 6 mo before the beginning of the study. H pylori infection was confirmed by positive results in both rapid urease test and histological examination, while H pylori-negative patients were defined by the absence of bacteria in both tests.
Patients with gastric ulcers consisted of 7 women and 8 men, with a mean age of 44.4 years (range 18-71 years). All peptic ulcers were located in the antrum and lesions were at the Sakita A1-A2 stages. After biopsy specimens were obtained, all patients were submitted to eradication therapy consisting of a 2 wk course of lansoprazole 30 mg, amoxycillin 1 g, and clarithromycin 500 mg given twice daily. Control endoscopy with gastric biopsies was undertaken at least 60 d after completion of eradication therapy.
As controls, biopsy samples were obtained from benign normal mucosa of consecutive patients undergoing diagnostic upper endoscopy for various clinical indications. Patients with H pylori-positive gastritis (without peptic ulcer) comprised 10 women and 4 men, with a mean age of 33.9 years (range: 20-58 years). The group of H pylori-negative patients consisted of 5 women and 9 men, with a mean age of 39.1 years (range: 20-63 years).
The study protocol was approved by the Ethical Committee of the University Hospital, Federal University of Rio de Janeiro, and informed consent was obtained from all patients.
Gastric biopsy specimens were endoscopically obtained from the antrum (2 cm away from the pyloric ring) of all patients in the study. From patients with gastric ulcer biopsy samples were collected from the antrum both at the ulcer margins and at the opposite wall of the lesion.
Biopsy specimens were taken for urease test, histology, and immunohistochemical studies. One specimen was used for the urease test. Two specimens were fixed in 10% normal saline, embedded in paraffin, cut into 5 μm sections and stained with hematoxylin and eosin (HE) and with Giemsa for histopathological examination. Another two antral specimens were immediately covered with Tissue Tek O.C.T. compound (Milles Scientific Laboratories Ltd, Naperville, IL, USA) and snap frozen in isopentane in a liquid nitrogen bath. These specimens were subsequently stored at -80°C before processing, and cut into 5 µm sections in a cryostat maintained at -20°C. Tissue sections were mounted onto slides pre-treated with poly-L-lysine (Sigma Chemical Co., St Louis, MO, USA), air-dried and fixed for 10 min in acetone at room temperature.
Formalin-fixed gastric sections were stained with hematoxylin and eosin for the evaluation of gastritis and with Giemsa stain to detect H pylori. The histological assessment of inflammation was performed according to the updated Sidney system[18]. Briefly, the parameters evaluated were inflammation (mononuclear leukocyte infiltration) and activity (polymorphonuclear neutrophil infiltration) of gastritis, density of H pylori colonization, atrophy, and intestinal metaplasia, which were graded as absent (0), mild (1), moderate (2), and severe (3), respectively.
For this set of experiments, frozen sections were used to characterize different cell subsets and for the study of apoptosis, which were performed by using the indirect immunoperoxidase technique.
Immunohistochemical staining was carried out using the following primary mouse monoclonal antibodies: anti-CD3 (1:50), anti-CD68 (1:50), anti-CD20 (1:50), anti-CD95/APO-1 (1:50) (Dako A/S, Glostrup, Denmark), anti-Fas ligand (1:1000), and anti-perforin (1:100) (Pharmingen, San Diego, CA, USA).
Briefly, frozen sections were immersed in 3% hydrogen peroxide in methanol for 10 min to block endogenous peroxidase activity. After being rinsed in phosphate buffered saline (PBS) containing 0.5% Tween 20 for 10 min, tissue sections were incubated with non-immune horse serum for 30 min and, subsequently, with the respective monoclonal antibody in a humidified chamber overnight, at room temperature. Two sections from each sample were incubated with either PBS alone or mouse monoclonal IgG1 (concentration-matched) (Dako A/S, Glostrup, Denmark) and served as negative controls. After being rinsed in PBS for 10 min, all tissue sections were incubated for 30 min with a goat anti-mouse peroxidase conjugate (1:200) (Zymed Laboratories, Inc., San Francisco, CA, USA).
To determine apoptosis, fragmented DNA was stained by the terminal deoxynucleotidyltransferase (TdT)-mediated dUDP-biotin nick end labelling (TUNEL) assay, with the TACSTM TdT kit-in situ apoptosis detection kit (R&D Systems, Minneapolis, MN, USA). Frozen sections were first incubated with proteinase K solution for 15 min at room temperature, and then immersed in hydrogen peroxide to block endogenous peroxidase activity, as described above. After washing, slides were incubated with the TdT labeling buffer for 5 min. This step was followed by the incubation with the labeling reaction mix containing TdT enzyme for 1 h at 37°C. The biotinylated nucleotides incorporated into DNA fragments were detected using streptavidin horseradish peroxidase conjugate. A second section of each sample, incubated without TdT enzyme, constituted the negative controls. Positive controls were prepared by treating samples with TACS-nuclease.
After being rinsed in PBS, all sections were developed with a solution containing hydrogen peroxide and diaminobenzidine. Preparations were lightly counterstained in Harris’s hematoxylin, dehydrated, and mounted in Permount (Fisher Scientific, Pittsburgh, PA, USA).
Morphologically preserved TUNEL-positive cells and apoptotic bodies were referred to as apoptotic cells and determined using predefined measurements in the computer-assisted image analyser in conjunction with careful evaluation of morphologic criteria.
In a double direct or indirect immunofluorescence study, the frozen sections were incubated overnight at 4°C with 2.5% bovine serum albumin (BSA), 2.0% skimmed milk, 8.0% fetal calf serum (FCS) blocking buffer under shaking. The sections were rinsed once with PBS and 0.05% Tween 20 and then incubated with appropriately diluted primary antibodies in PBS and 1.0% FCS for 1 h in a humidified atmosphere at 37°C. The primary antibodies used were the anti-CD3 FITC (1:50), anti-CD68 FITC (1:50), anti-CD20 FITC (1:50), anti-CD95 (Fas/APO-1) R-PE (1:50) (Dako A/S, Glostrup, Denmark), anti-Fas ligand (1:1000), and the anti-perforin R-PE (1:100) (Pharmingen, San Diego, CA, USA). After rinsing three times in PBS containing 0.05% Tween 20 for 5 min each, tissue sections incubated with anti-Fas ligand were incubated for 1 h with TRITC-conjugated Fab fraction of goat anti-mouse IgG antibody (Dako A/S, Glostrup, Denmark), as appropriate for indirect immunolabelling. Slides were rinsed three times and mounted in an anti-fading medium containing buffered glycerol and p-phenylenediamine (Sigma Chemical Co., St Louis MO, USA), and then observed with a Zeiss LSM 510 META confocal laser scanning microscope. At least four representative images from each slide were captured at distinct excitation wavelengths (488 nm for FITC conjugate, and 543 nm for TRITC or R-PE conjugates), creating excitation lambda stacks. Using the META detector, excitation lambda stacks were separated into individual images corresponding to the signal from distinct dyes, eliminating autofluorescence and crosstalk between fluorochromes. Two sections from each sample were incubated with either PBS alone or FITC- or TRITC-conjugated anti-mouse IgG antibody, and served as negative controls.
Quantitative analysis of tissue sections (under light microscope at × 400 magnification) and of captured images of immunofluorescence (under confocal laser scanning microscope) was carried out using a computer-assisted image analyser (Image-Pro Plus Version 4.1 for Windows, Media Cybernetics, LP, Silver Spring, MD, USA). Any epithelial and lamina propria cells exhibiting identifiable reactivity distinct from background were regarded as positive.
In the immunoperoxidase studies, percentages of the different cell subsets or apoptotic cells were defined by the number of immunoreactive cells in relation to total cells (immunoreactive and non-immunoreactive cells) in the lamina propria per millimetre squared (counted in at least 10 different areas), or in at least 500 epithelial cells in the crypts and in the surface epithelium of longitudinally sectioned gastric pits.
In the double immunofluorescence studies, the immunoreactive cells were counted in the lamina propria per millimetre squared. Co-localization was defined as the numbers of double-positive cells (yellowish colour) in relation to one of the single-positive cells (green colour), in the lamina propria per millimetre squared (counted in at least 4 different areas). Co-expression was further confirmed with confocal LSM co-localization tool (data not shown). Two independent observers who were unaware of the patients’ data examined all tissue sections and captured images.
Statistical analysis was performed using the statistical software SPSS for Windows (Version 10.0.1, SPSS Inc., 1989-1999, USA). Statistical differences among the experimental groups were evaluated with the one-way ANOVA test in which pairwise multiple comparisons were carried out using the Dunnett’s T3 test, and the Wilcoxon signed rank test for comparisons between patients with gastric ulcers (before and after treatment; gastric antrum and marginal zone of the ulcer). Correlations between the densities of positive cells measured by immunohistochemistry and the co-localization studies were assessed using the Spearman rank correlation coefficient. Values are expressed as medians (1st quartile, 3rd quartile). The level of significance was set at P < 0.05.
Higher inflammatory scores were found in the mucosa of gastritis and GU patients infected with H pylori compared to the normal mucosa of control patients free of infection and the mucosa of the same patients after eradication therapy. Scores in the GU patient group were 5.0 (3.3-5.0) at the ulcer margins, while the antrum scored 4.0 (3.0-5.0) before and 2.0 (1.0-2.0) after eradication therapy. In the antrum of untreated H pylori-associated gastritis the score was 3.0 (3.0-3.0), and in the control group of normal non-infected mucosa the score was 0.5 (0.0-1.0). Atrophy and intestinal metaplasia were not detected in any specimens.
A significantly higher number of T cells was found at both the ulcer margin and antrum of patients with GU (12.7% and 17.7%, respectively) compared with the normal non-infected control group (7.1%) (P = 0.027 and P < 0.001, respectively). After eradication therapy, rates of T cells in the antrum significantly decreased from 17.7% to 13.1% (P = 0.046). The number of macrophages was also significantly higher in the antrum of GU patients before treatment (12.0%) compared with that of the control group (6.9%) (P = 0.029). In addition, in the GU patient group, the number of macrophages in the ulcer margin and antrum (11.1% and 12.0%, respectively) decreased after eradication therapy (6.8%) (P = 0.028 and P = 0.043, respectively). In H pylori-positive patients with gastritis, the number of T cells (13.9%) and macrophages (12.7%) was not significantly different from those in the GU and control group. The proportions of B cells were similar in all groups.
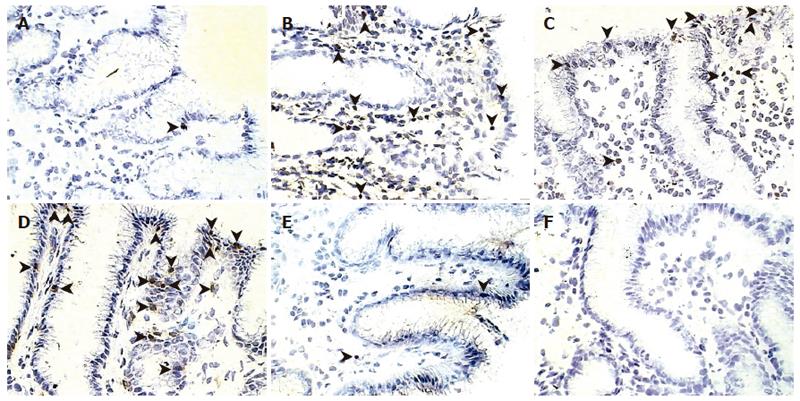
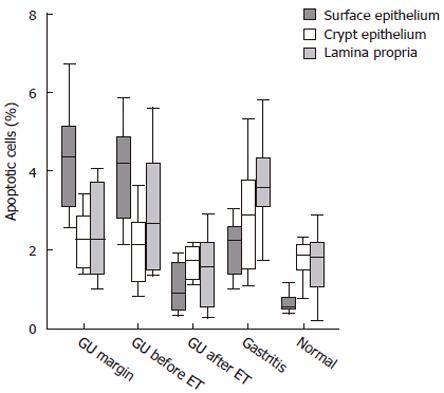
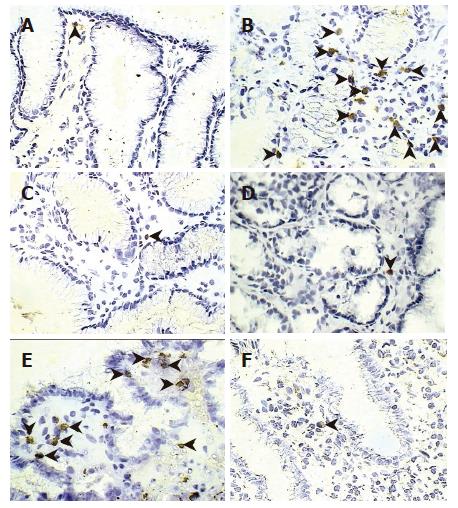
In the GU group, the location of epithelial cell apoptosis was predominant in the superficial layer, both at the ulcer margin and the opposite wall of the antrum. In contrast, in the infected mucosa from patients with gastritis, apoptosis was more frequent in the crypts (Figure 1). When the proportion of apoptotic cells was assessed, patients who were H pylori-positive contained a significantly (P < 0.03) greater number of apoptotic cells in the mucosa, regardless of the diagnosis, than in the same group after eradication and in non-infected controls (Figure 2). In the GU group, the number of apoptotic cells was significantly (P < 0.003) higher than in the gastritis group. The proportion of apoptotic cells in the crypts was similar in all groups.
When the lamina propria was assessed, apoptosis of inflammatory cells was seen more frequently (P < 0.01) in the mucosa of patients with gastritis compared to that of treated GU patients and non-infected controls (Figure 2). A significant correlation was found between the inflammatory scores and the number of apoptotic cells in the surface epithelium (r = 0.71, P < 0.001), but not the crypt epithelium or the lamina propria.
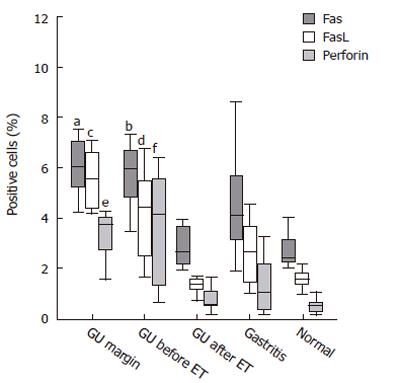
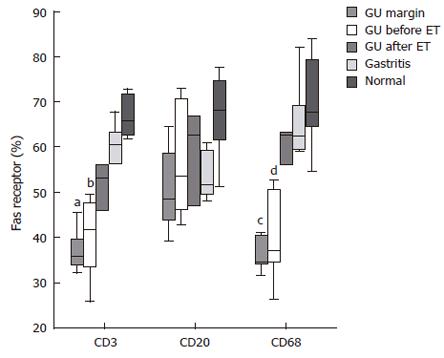
Immunohistochemistry revealed that Fas was constitutively expressed by all epithelial cells, with no differences detected among all groups. In the lamina propria, the percentage of Fas-positive cells was significantly increased in patients with GU, both at the ulcer margin and the antral mucosa, compared with the mucosa of the same patients after eradication therapy (P < 0.01) and those of non-infected controls (P < 0.04) (Figure 3). A significant correlation was found between the number of Fas-positive cells within the lamina propria and inflammatory scores (r = 0.62, P < 0.001).
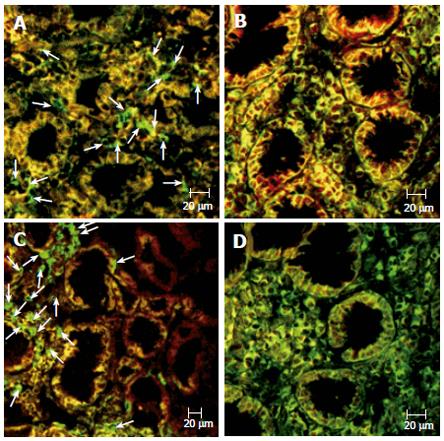
Next, we tried to identify the type of inflammatory cells co-expressing Fas by confocal double immunofluorescence. The expression of Fas by lamina propria T cells from the ulcer margin and the untreated antral mucosa was significantly reduced compared with both cells in the antrum after treatment (P < 0.05) and in non-infected controls (P < 0.02) (Figures 4 and 5). Essentially the same results were obtained when co-expression of Fas by macrophages was evaluated (Figures 4 and 5). In regard to B cells, no significant differences were found in the gastric mucosa among all groups.
Immunostaining for FasL was predominantly located in the lamina propria, and rarely seen in intraepithelial lymphocytes. The percentage of FasL-positive cells was increased in the mucosa of patients with GU, both at the ulcer margin and the antrum before treatment, compared with that observed after treatment and in the non-infected control group (Figures 3 and 6). We further investigated the co-localization of FasL in the gastric lamina propria and, as expected, essentially all FasL-positive cells co-expressed CD3 (data not shown). Of note, a significant correlation existed between the expression of FasL by gastric mucosa T cells and the percentages of apoptotic cells in the surface epithelium (r = 0.71; P < 0.001) and the histological inflammatory scores (r = 0.63, P < 0.001).
Immunostaining for perforin was essentially restricted to the lamina propria, with occasional staining in intraepithelial lymphocytes. Perforin-positive cells were significantly more frequent in the lamina propria of patients with GU, both at the ulcer margin and the untreated antrum, compared to the antrum of gastritis patients (P < 0.04), treated GU patients (P < 0.01), and normal non-infected controls (P < 0.01) (Figures 3 and 6). Confocal microscopy confirmed that all perforin-positive cells were also CD3-positive (data not shown). A significant correlation was detected between the number of perforin-expressing cells in the gastric mucosa and the percentage of apoptotic cells in the surface epithelium (r = 0.61; P < 0.001) as well as the inflammatory scores (r = 0.59, P < 0.001).
In the current study, we detected increased rates of apoptosis in the surface epithelium of the gastric mucosa infected with H pylori, predominantly of GU patients. Increased rates of apoptosis were also observed among lamina propria mononuclear cells of patients with H pylori-associated gastritis. The expression of Fas, FasL, and perforin in the H pylori-infected gastric mucosa was increased compared with normal non-infected controls and with patients after eradication therapy. The higher expression of apoptosis-related molecules was found in the antral mucosa of patients with gastric ulcer, and this result was significantly correlated with the levels of apoptosis at the surface epithelium. In addition, we showed for the first time an in situ analysis of the densities and co-localization of apoptosis-related molecules in the antral mucosa from patients with gastric ulcer, before and after eradication therapy, and both at the ulcer margin and at the opposite antral wall. The results demonstrated that there was a reduction in the expression of Fas by both T cells and macrophages in the antral lamina propria of GU patients.
Although H pylori-related gastritis continues to constitute one of the most common human infections, only a subset of patients will develop severe complications of the infection such as peptic ulcer disease. Histological findings of the chronically infected stomach reveal a dense cellular infiltration throughout the mucosa, whereas H pylori locates predominantly in the overlying mucous layer. Several studies have provided evidence that activated T-helper type 1 cells are increased in H pylori-associated gastritis[6,7], and the consequent pro-inflammatory stimuli have been associated with the sensitization and induction of epithelial apoptosis[9,10]. However, in normal conditions epithelial apoptosis appears to be an infrequent event in the gastric mucosa. In the present work, the results of in situ analysis suggest that increased rates of epithelial apoptosis are related to H pylori infection, in agreement with previous in vivo and in vitro studies[8,19,20]. Significantly higher rates were found in patients with peptic ulcer and decreased significantly after eradication therapy reaching normal levels. In addition, the localization of epithelial apoptosis differed among groups. In the peptic ulcer group apoptotic cells were found predominantly in the superficial epithelium, whereas in the gastritis group the phenomenon was mostly located at the crypts. Distinct localization patterns of epithelial apoptosis seem to be in accordance with either the most likely outcome of peptic ulcer whenever surface epithelial damage prevails or with atrophy in chronic H pylori-associated gastritis.
The induction of macrophage apoptosis has been considered as a possible method employed by multiple pathogens to escape the host immune response[21]. In fact, apoptosis of immunocompetent cells such as macrophages[22], and B cells[23] has been reported in association with H pylori infection. Furthermore, H pylori may also evade the host immune response through the impairment of antigen-specific T cell proliferation[24], or inducing T cell apoptosis[25]. Therefore, at least in theory, apoptosis of immunocompetent cells is likely to decrease the local inflammatory process, but on the other hand it may reduce the effective immune response against H pylori as well. Apoptosis of mononuclear leukocytes in the gastric mucosa infected by H pylori was also reported after eradication therapy[26]. Although in the present study the rates of apoptosis in the lamina propria after eradication therapy were comparable to normal controls, the mucosa showed a concomitant reduction in the number of inflammatory cells. Thus, it was possible that the number of those cells could have been reduced by induction of apoptosis after therapy. However, if this was the case in our study, the effect would be likely to have decreased to the normal levels by the time of the follow-up biopsy. On the other hand, a relative resistance to H pylori-induced apoptosis was detected in neutrophils[27], and dendritic cells[28] in vitro. In this study, we found significantly increased rates of apoptosis in the lamina propria mononuclear cells of patients with H pylori-related gastritis compared to the non-infected mucosa. The fact that this phenomenon has not been observed in the antral mucosa of GU patients may reflect the existence of a defect in the control of local host responses. It is possible that apoptosis of lamina propria mononuclear cells might constitute a mechanism by which the host may down-regulate the inflammatory response associated with chronic H pylori infection.
Apoptosis constitutes the primary mechanism of target-cell destruction mediated by cytotoxic T lymphocytes, and one of the pathways involved in this process is the Fas/FasL. Increased rates of apoptosis reported in H pylori-infected gastric mucosa has been associated with the Fas/FasL pathway[15,17,29]. In vitro studies have demonstrated that the sensitivity of gastric epithelial cells to Fas-mediated apoptosis could be increased by the up-regulation of the Fas-receptor expression consequent to H pylori infection[14] or triggered by inflammatory cytokines[30]. In addition, it was demonstrated that increased expression of Fas by gastric epithelium renders cells sensitive to apoptosis by adjacent FasL-expressing T cells[16].
In contrast to T cell responses, the epithelial expression of FasL is low or undetectable[31], and blocking the Fas receptor cannot protect those cells from apoptosis[29]. Therefore, it seems that H pylori may be capable of inducing apoptosis in different cell lineages using diverse but possibly overlapping mechanisms. Nevertheless, in T cells the response appears to be mostly dependent on Fas/FasL interactions[25]. In the present study, we found that the levels of FasL-positive cells in the lamina propria were positively correlated with surface epithelial apoptosis. However, increased numbers of FasL-positive cells were present in all patients infected with H pylori, without any significant difference between the results of gastritis and GU patients. These findings suggest that FasL might participate in the inflammatory process associated with H pylori infection, but may not be directly and specifically related to ulcerogenesis.
Fas/FasL-mediated apoptosis is regulated mostly by the cellular expression of both molecules, since Fas is activated almost exclusively by the membrane-bound form of its natural ligand, FasL[32]. However, cellular mechanisms that control the expression of Fas and FasL appear to be independent. Fas is more universally distributed and is thought to be expressed later in the course of cellular activation[33], while the up-regulation of FasL is regarded as an early event of T-cell activation[34]. Hence, the expression of Fas on T-cells and other lamina propria mononuclear cells could have an effect of down-regulation of gastrointestinal immune responses through apoptosis, upon binding with FasL. In accordance with the findings of the present study, Fas is expressed constitutively in the epithelial cells of the human stomach[30], where it has been considered as a key molecule in the control of apoptosis. In the lamina propria infected with H pylori we observed increased numbers of Fas-expressing cells. However, since the numbers of inflammatory cells were also significantly increased in the infected mucosa, the expression of Fas could actually be proportionally altered. In fact, the expression of Fas was shown to be reduced by both T cells and macrophages in the lamina propria of GU patients. Taken together, these results suggest that the increased numbers of FasL-expressing cells might result in increased epithelial cytotoxicity not paralleled by lamina propria mononuclear cell apoptosis mediated by the Fas/FasL system in GU patients. It is hypothesized that an uncoordinated regulation of Fas and FasL could possibly result in decreased apoptosis of inflammatory cells, including auto-reactive cells in the lamina propria of GU patients.
In conjunction with T cells, macrophages are recognized as fundamental elements of the chronic inflammatory process of GU. Therefore, it is reasonable to suppose that mechanisms to eliminate activated macrophages should also be required for the maintenance of peripheral tolerance. Results from an in vitro study demonstrated that in addition to T-T and T-B lymphocyte cytotoxicity, the Fas/FasL system is also capable of mediating T-macrophage cytotoxicity[35]. Hence, in the current study the reduced expression of Fas on macrophages shown in the gastric mucosa from patients with gastric ulcer could possibly explain the accumulation and persistence of those cells in sites of gastric inflammation.
Increased levels of granzyme B mRNA, introduced through the action of perforin, have been found in cells adjacent to apoptotic epithelial cells of specimens obtained from the marginal zone of active gastric and duodenal ulcers. But interestingly, in that study the findings were shown also in patients with peptic ulcer in the absence of H pylori infection[26]. In the current study, perforin was detected at increased rates in GU patients, both at margins and tissues away from the lesion, compared to both non-infected controls and H pylori-related gastritis. Moreover, the levels of perforin were significantly correlated with the rates of surface epithelial apoptosis. This fact may support the contribution of distinct pathways to the cell-mediated cytotoxicity against innocent bystander targets such as the gastric epithelium, but possibly with a greater role for perforin in the development of peptic ulcer disease. On the other hand, the positive correlation between the rates of perforin-expressing cells and apoptotic cells in the lamina propria of patients with H pylori-associated gastritis may indicate the enhancement of an alternative pathway as a mechanism to circumvent the resistance to apoptosis and chronic mucosal inflammation.
In conclusion, the results indicate that increased apoptosis in the surface epithelium may contribute to the pathologic process of gastric ulcer. The lamina propria Fas/FasL expression may reflect the state of inflammation secondary to H pylori infection. However, the consistently increased percentage of perforin-expressing cells in the antrum of GU patients suggests a distinct role of perforin in ulcerogenesis.
S- Editor Wang J L- Editor Zhu LH E- Editor Liu WF
| 1. | Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311-1315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3302] [Cited by in RCA: 3265] [Article Influence: 79.6] [Reference Citation Analysis (1)] |
| 2. | Graham DY, Lew GM, Evans DG Jr, Evans DJ, Klein PD. Effect of triple therapy (antibiotics plus bismuth) on duodenal ulcer healing. A randomized controlled trial. Ann Intern Med. 1991;115:266-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 141] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 3. | Parsonnet J, Hansen S, Rodriguez L, Gelb AB, Warnke RA, Jellum E, Orentreich N, Vogelman JH, Friedman GD. Helicobacter pylori infection and gastric lymphoma. N Engl J Med. 1994;330:1267-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1287] [Cited by in RCA: 1229] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 4. | Blaser MJ, Perez-Perez GI, Kleanthous H, Cover TL, Peek RM, Chyou PH, Stemmermann GN, Nomura A. Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. 1995;55:2111-2115. [PubMed] |
| 5. | Ernst P. Review article: the role of inflammation in the pathogenesis of gastric cancer. Aliment Pharmacol Ther. 1999;13 Suppl 1:13-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 153] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 6. | Lehmann FS, Terracciano L, Carena I, Baeriswyl C, Drewe J, Tornillo L, De Libero G, Beglinger C. In situ correlation of cytokine secretion and apoptosis in Helicobacter pylori-associated gastritis. Am J Physiol Gastrointest Liver Physiol. 2002;283:G481-G488. [PubMed] |
| 7. | Bamford KB, Fan X, Crowe SE, Leary JF, Gourley WK, Luthra GK, Brooks EG, Graham DY, Reyes VE, Ernst PB. Lymphocytes in the human gastric mucosa during Helicobacter pylori have a T helper cell 1 phenotype. Gastroenterology. 1998;114:482-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 425] [Article Influence: 15.7] [Reference Citation Analysis (1)] |
| 8. | Moss SF, Calam J, Agarwal B, Wang S, Holt PR. Induction of gastric epithelial apoptosis by Helicobacter pylori. Gut. 1996;38:498-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 313] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 9. | Fan X, Crowe SE, Behar S, Gunasena H, Ye G, Haeberle H, Van Houten N, Gourley WK, Ernst PB, Reyes VE. The effect of class II major histocompatibility complex expression on adherence of Helicobacter pylori and induction of apoptosis in gastric epithelial cells: a mechanism for T helper cell type 1-mediated damage. J Exp Med. 1998;187:1659-1669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 147] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 10. | Wagner S, Beil W, Westermann J, Logan RP, Bock CT, Trautwein C, Bleck JS, Manns MP. Regulation of gastric epithelial cell growth by Helicobacter pylori: offdence for a major role of apoptosis. Gastroenterology. 1997;113:1836-1847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 212] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 11. | Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4724] [Cited by in RCA: 4686] [Article Influence: 156.2] [Reference Citation Analysis (0)] |
| 12. | Sträter J, Wellisch I, Riedl S, Walczak H, Koretz K, Tandara A, Krammer PH, Möller P. CD95 (APO-1/Fas)-mediated apoptosis in colon epithelial cells: a possible role in ulcerative colitis. Gastroenterology. 1997;113:160-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 183] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 13. | Itoh J, de La Motte C, Strong SA, Levine AD, Fiocchi C. Decreased Bax expression by mucosal T cells favours resistance to apoptosis in Crohn's disease. Gut. 2001;49:35-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 97] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Jones NL, Day AS, Jennings HA, Sherman PM. Helicobacter pylori induces gastric epithelial cell apoptosis in association with increased Fas receptor expression. Infect Immun. 1999;67:4237-4242. [PubMed] |
| 15. | Houghton J, Korah RM, Condon MR, Kim KH. Apoptosis in Helicobacter pylori-associated gastric and duodenal ulcer disease is mediated via the Fas antigen pathway. Dig Dis Sci. 1999;44:465-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 42] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Wang J, Fan X, Lindholm C, Bennett M, O'Connoll J, Shanahan F, Brooks EG, Reyes VE, Ernst PB. Helicobacter pylori modulates lymphoepithelial cell interactions leading to epithelial cell damage through Fas/Fas ligand interactions. Infect Immun. 2000;68:4303-4311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 67] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Ishihara S, Fukuda R, Kawashima K, Moriyama N, Suetsugu H, Ishimura N, Kazumori H, Kaji T, Sato H, Okuyama T. T cell-mediated cytotoxicity via Fas/Fas ligand signaling in Helicobacter pylori-infected gastric corpus. Helicobacter. 2001;6:283-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3221] [Cited by in RCA: 3555] [Article Influence: 122.6] [Reference Citation Analysis (3)] |
| 19. | Israel DA, Salama N, Arnold CN, Moss SF, Ando T, Wirth HP, Tham KT, Camorlinga M, Blaser MJ, Falkow S. Helicobacter pylori strain-specific differences in genetic content, identified by microarray, influence host inflammatory responses. J Clin Invest. 2001;107:611-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 237] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 20. | Satoh K, Kawata H, Tokumaru K, Kumakura Y, Ishino Y, Kawakami S, Inoue K, Kojima T, Satoh Y, Mutoh H. Change in apoptosis in the gastric surface epithelium and glands after eradication of Helicobacter pylori. Dig Liver Dis. 2003;35:78-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Menaker RJ, Ceponis PJ, Jones NL. Helicobacter pylori induces apoptosis of macrophages in association with alterations in the mitochondrial pathway. Infect Immun. 2004;72:2889-2898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 63] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Gobert AP, Cheng Y, Wang JY, Boucher JL, Iyer RK, Cederbaum SD, Casero RA Jr, Newton JC, Wilson KT. Helicobacter pylori induces macrophage apoptosis by activation of arginase II. J Immunol. 2002;168:4692-4700. [PubMed] |
| 23. | Reinacher-Schick A, Petrasch S, Burger A, Suerbaum S, Kunstmann E, Schmiegel W. Helicobacter pylori induces apoptosis in mucosal lymphocytes in patients with gastritis. Z Gastroenterol. 1998;36:1021-1026. [PubMed] |
| 24. | Paziak-Domańska B, Chmiela M, Jarosińska A, Rudnicka W. Potential role of CagA in the inhibition of T cell reactivity in Helicobacter pylori infections. Cell Immunol. 2000;202:136-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 25. | Wang J, Brooks EG, Bamford KB, Denning TL, Pappo J, Ernst PB. Negative selection of T cells by Helicobacter pylori as a model for bacterial strain selection by immune evasion. J Immunol. 2001;167:926-934. [PubMed] |
| 26. | Ohara T, Morishita T, Suzuki H, Masaoka T, Ishii H. Perforin and granzyme B of cytotoxic T lymphocyte mediate apoptosis irrespective of Helicobacter pylori infection: possible act as a trigger of peptic ulcer formation. Hepatogastroenterology. 2003;50:1774-1779. [PubMed] |
| 27. | Kim JS, Kim JM, Jung HC, Song IS, Kim CY. Inhibition of apoptosis in human neutrophils by Helicobacter pylori water-soluble surface proteins. Scand J Gastroenterol. 2001;36:589-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Galgani M, Busiello I, Censini S, Zappacosta S, Racioppi L, Zarrilli R. Helicobacter pylori induces apoptosis of human monocytes but not monocyte-derived dendritic cells: role of the cag pathogenicity island. Infect Immun. 2004;72:4480-4485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 29. | Rudi J, Kuck D, Strand S, von Herbay A, Mariani SM, Krammer PH, Galle PR, Stremmel W. Involvement of the CD95 (APO-1/Fas) receptor and ligand system in Helicobacter pylori-induced gastric epithelial apoptosis. J Clin Invest. 1998;102:1506-1514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 179] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 30. | Houghton J, Macera-Bloch LS, Harrison L, Kim KH, Korah RM. Tumor necrosis factor alpha and interleukin 1beta up-regulate gastric mucosal Fas antigen expression in Helicobacter pylori infection. Infect Immun. 2000;68:1189-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Möller P, Walczak H, Reidl S, Sträter J, Krammer PH. Paneth cells express high levels of CD95 ligand transcripts: a unique property among gastrointestinal epithelia. Am J Pathol. 1996;149:9-13. [PubMed] |
| 32. | Ju ST, Panka DJ, Cui H, Ettinger R, el-Khatib M, Sherr DH, Stanger BZ, Marshak-Rothstein A. Fas(CD95)/FasL interactions required for programmed cell death after T-cell activation. Nature. 1995;373:444-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1159] [Cited by in RCA: 1200] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 33. | Miyawaki T, Uehara T, Nibu R, Tsuji T, Yachie A, Yonehara S, Taniguchi N. Differential expression of apoptosis-related Fas antigen on lymphocyte subpopulations in human peripheral blood. J Immunol. 1992;149:3753-3758. [PubMed] |
| 34. | Brunner T, Mogil RJ, LaFace D, Yoo NJ, Mahboubi A, Echeverri F, Martin SJ, Force WR, Lynch DH, Ware CF. Cell-autonomous Fas (CD95)/Fas-ligand interaction mediates activation-induced apoptosis in T-cell hybridomas. Nature. 1995;373:441-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1005] [Cited by in RCA: 1044] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 35. | Ashany D, Song X, Lacy E, Nikolic-Zugic J, Friedman SM, Elkon KB. Th1 CD4+ lymphocytes delete activated macrophages through the Fas/APO-1 antigen pathway. Proc Natl Acad Sci USA. 1995;92:11225-11229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 97] [Article Influence: 3.2] [Reference Citation Analysis (0)] |