Published online Oct 7, 2006. doi: 10.3748/wjg.v12.i37.6021
Revised: March 25, 2005
Accepted: April 26, 2005
Published online: October 7, 2006
AIM: To investigate the relationship between mucin 6 (MUC6) VNTR length and H pylori infection.
METHODS: Blood samples were collected from patients visiting the Can Tho General Hospital for upper gastrointestinal endoscopy. DNA was isolated from whole blood, the repeated section was cut out using a restriction enzyme (PvuII) and the length of the allele fragments was determined by Southern blotting. H pylori infection was diagnosed by 14C urea breath test. For analysis, MUC6 allele fragment length was dichotomized as being either long (> 13.5 kbp) or short (≤ 13.5 kbp) and patients were classified according to genotype [long-long (LL), long-short (LS), short-short (SS)].
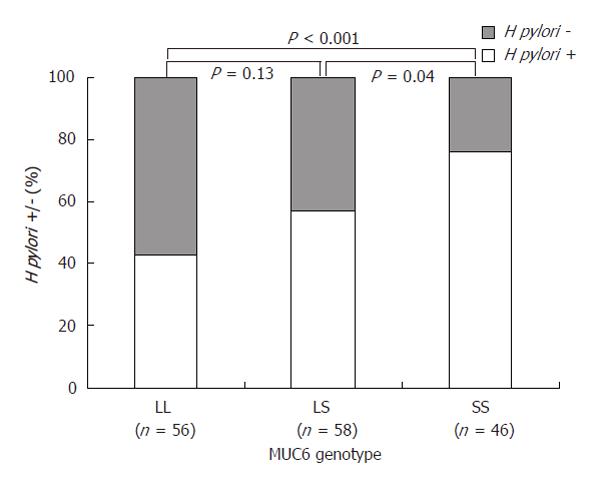
RESULTS: 160 patients were studied (mean age 43 years, 36% were males, 58% H pylori positive). MUC6 PvuII-restricted allele fragment lengths ranged from 7 to 19 kbp. Of the patients with the LL, LS, SS MUC6 genotype, 43% (24/56), 57% (25/58) and 76% (11/46) were infected with H pylori, respectively (P = 0.003).
CONCLUSION: Short MUC6 alleles are associated with H pylori infection.
- Citation: Nguyen TV, Janssen MJ, Gritters P, Morsche RHT, Drenth JP, Asten HV, Laheij RJ, Jansen JB. Short mucin 6 alleles are associated with H pylori infection. World J Gastroenterol 2006; 12(37): 6021-6025
- URL: https://www.wjgnet.com/1007-9327/full/v12/i37/6021.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i37.6021
H pylori has the unique ability to colonize the human stomach. Infection with H pylori invariably leads to gastritis and in many instances to peptic ulcer disease[1]. Additionally, H pylori infection has been associated with gastric cancer[2]. It is a common infection throughout the world, with prevalence ranging from below 20% in developed countries to over 80% in developing countries. Some risk factors for H pylori infection have been identified, such as low socio-economic status or poor hygiene[3]. However, there is remarkable inter-individual variability in susceptibility to the infection that cannot be explained by differences in environmental factors.
Another factor that may be related to H pylori infection susceptibility is the composition of the mucus gel layer in the stomach, in which H pylori resides. This layer protects the underlying epithelium from acid, proteases, mechanical trauma, and pathogenic micro-organisms and its main constituents are high molecular weight glycoproteins named mucins. These mucins consist of a polypeptide backbone with O-linked oligosaccharide side chains, which largely determine the properties of the mucins[4]. Interestingly, there is substantial inter-individual variation in the number of these side chains. This is caused by a variable number tandem repeat (VNTR) polymorphism in the genes encoding for the mucins. VNTRs consist of repeated DNA sequences and the number of repeats is highly variable. The resulting repeated amino acid sequences are located in the central part of the mucin polypeptide backbone, to which the oligosaccharide side chains are attached[5]. Therefore, this polymorphism leads to the production of mucin polypeptides that substantially differ in both length and glycosylation[6,7]. Thus, this VNTR polymorphism may affect the protective properties of the mucins and consequently the susceptibility to H pylori infection.
Normal gastric mucosa is characterized by the expression of mucins MUC1, MUC5AC and MUC6. MUC1 is of the membrane-bound type, whereas MUC5AC and MUC6 are of the secreted, gel-forming type[8]. MUC6 and MUC1 show extensive VNTR variation, MUC5AC only moderate[6,9]. Furthermore, the length of the repeated sequence differs: a single MUC6 tandem repeat sequence consists of 507 base pairs whereas single MUC1 and MUC5AC tandem repeat sequences consist of only 60 and 24 base pairs, respectively[6]. Therefore, the VNTR polymorphism has the most profound impact on allele length and protein structure of MUC6.
Despite the significant structural consequences of these VNTR polymorphisms, few studies investigated their pathophysiological consequences. In a study comparing gastric cancer patients with healthy blood donors, shorter VNTR sections were associated with gastric cancer for MUC6[10] and MUC1[11]. This effect may be mediated by an altered susceptibility to H pylori infection which is an important factor in gastric carcinogenesis, since Vinall et al[9] showed that short VNTR sections were associated with H pylori infection for MUC1. However, there are no data available regarding the relationship between H pylori infection and VNTR polymorphism in MUC6, which is abundant in the stomach and has the most extensive VNTR variation[12].
Therefore, the aim of this study was to investigate the hypothesis that susceptibility to H pylori infection is related to MUC6 VNTR length. We studied a sample of 160 patients referred for upper gastrointestinal endoscopy and found that patients with short MUC6 allele fragments have a significantly higher risk of being infected with H pylori, which suggests that mucin 6 protein length modifies susceptibility to H pylori.
From September to December 2003, all patients with upper gastrointestinal symptoms visiting the Gastroenterology Outpatient Clinic of the Can Tho General Hospital for upper gastrointestinal endoscopy were asked to participate in this study. Patients who had not been treated for H pylori infection in the past and who gave written informed consent were included in the study. At baseline, data regarding age, gender, smoking habits and alcohol consumption were registered.
All patients had a 14C urea breath test (HeliprobeTM, Noster system AB, Stockholm, Sweden). Patients were not allowed to use proton pump inhibitors/H2-receptor antagonists or antibiotics during the two weeks preceding the breath testing. After an overnight fast patients took a HeliCapTM capsule (containing 1 μCi of 14C urea) with 50 mL of water. Ten minutes later, a breath sample was collected (BreathCardTM) and analyzed during 4 min. Measuring more than 50 counts was regarded as proof of H pylori infection, measuring fewer than 25 counts was regarded as proof of absence of H pylori infection[13].
Blood samples were collected for DNA isolation (PuregeneTM kit, Gentra systems, Minneapolis, USA). MUC6 allele fragment length was measured using Southern blot analysis. The DNA samples were digested with the PvuII restriction enzyme as described previously by Vinall et al[6]. This enzyme cuts just outside the tandem repeat domain and clearly reveals the MUC6 VNTR polymorphism. The resulting DNA fragments were separated by agarose gel electrophoresis (0.7% agarose in 0.04 mol/L Tris, 0.001 mol/L EDTA, pH 8.0) at 35 V for 18 h. We used λ-HindIII digest as a marker of DNA fragment length. DNA fragments were then transferred onto nylon membranes (Gene Screen PlusTM hybridization transfer membrane, Boston, USA). Afterwards, the nylon membranes were treated with ultraviolet radiation and prepared for hybridization by Church buffer. The probe, which consisted of two MUC6 tandem repeats, was produced by polymerase chain reaction with forward primer 5’-ACCTCTTTGGTGACTCCAATTA-3’ and reverse primer 5’-AACGTGAGTGGGAAGTGTGGT-3’ and randomly labelled with α-32PdCTP. The resulting PCR product was verified by sequencing. After 18 h of hybridization of the probe in 0.5 mol/L phosphate buffer containing 7% SDS and 0.001 mol/L EDTA, SSPE/SDS-solutions were used to remove non-specifically bound probe. Using the λ-HindIII digest as a reference, the individual MUC6 allele fragment lengths were calculated.
The primary outcome of this study was the presence or absence of H pylori infection. For analysis, MUC6 allele fragment length was dichotomized as being either short (≤ 13.5 kb) or long (> 13.5 kb) and patients were classified according to genotype [long-long (LL), long-short (LS), short-short (SS)]. Baseline characteristics for H pylori positive and negative patients were compared. MUC6 genotype and baseline characteristics were related to H pylori infection by means of unadjusted and adjusted logistic regression analyses, using the SAS® statistical software package (SAS Institute Inc., USA). Statistical significance was defined as a P < 0.05. Missing values were excluded from analysis.
During the study period, 160 patients [mean age 43 years, 58 (36%) males, 92 (58%) H pylori infected] were included. Table 1 shows that H pylori positive and negative patients were comparable for all baseline characteristics except age.
| H pylori positive H pylori negative | ||
| Characteristic | n (%) | n (%) |
| (n = 92) | (n = 68) | |
| Mean age (SD) (yr) | 46 (12)a | 39 (13) |
| Gender | ||
| Male | 29 (50) | 29 (50) |
| Female | 63 (62) | 39 (38) |
| Currently smoking | ||
| Yes | 22 (56) | 17 (44) |
| No | 70 (58) | 51(42) |
| Current alcohol consumption | ||
| Yes | 16 (55) | 13 (45) |
| No | 76 (58) | 55 (42) |
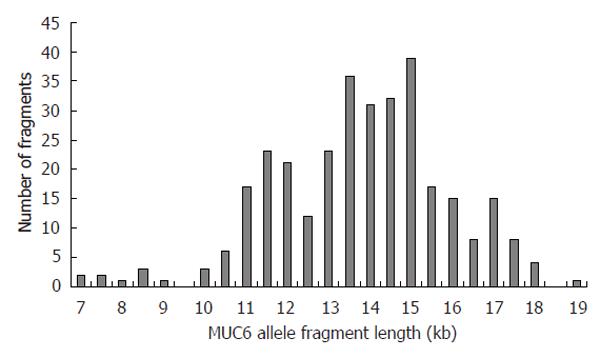
Using the restriction enzyme PvuII, a clear length polymorphism was detected. Minimum fragment length difference was 0.5 kb, reflecting the length of a single MUC6 tandem repeat that consists of 507 base pairs. MUC6 was found to be highly polymorphic with PvuII-restricted fragment lengths ranging from 7 to 19 kb [mean 13.8 kb (SD: 2)] (Figure 1).
Mean MUC6 allele fragment length was shorter for H pylori positive patients than for H pylori negative patients (13.4 vs 14.2, P = 0.001). Since there were too many different allele fragment lengths to analyse separately, MUC6 allele fragment length was dichotomized as being either short (≤13.5 kb) or long (> 13.5 kb). Furthermore, patients were grouped according to genotype [long-long (LL), long-short (LS), short-short (SS)] (Table 2).
| Factor | Unadjusted analysis | Adjusted analysis1 | ||
| Odds ratio | 95% CI | Odds ratio | 95% CI | |
| MUC6 genotype (SS vs LS/LL)a | 3.18 | 1.5-6.9 | 2.93 | 1.3-6.5 |
| Age group (> 45 vs ≤ 45 yr)a | 2.11 | 1.1-4.1 | 2.11 | 1.0-4.3 |
| Gender (male vs female) | 0.62 | 0.3-1.2 | 0.47 | 0.2-1.9 |
| Currently smoking | 0.94 | 0.5-2.0 | 1.46 | 0.5-4.3 |
| Current alcohol consumption | 0.89 | 0.4-2.0 | 1.39 | 0.5-3.9 |
Patients with two short allele fragments were more often infected with H pylori than patients with one long and one short allele fragment [Odds ratio (95% CI): 2.41 (1.0-5.7)] or patients with two long allele fragments [4.24 (1.8-10.0)]. Figure 2 shows that there seems to be a gradual increase in prevalence of H pylori infection from 43% (24/56) for patients with two long allele fragments, through 57% (25/58) for patients with one long and one short allele fragments, to 76% (11/46) for patients with two short allele fragments.
Additionally, Table 2 shows that of the other patient characteristics measured, only age was associated with
H pylori infection, and that the influence of MUC6 genotype remained virtually unchanged after adjusting for age group, gender, smoking and alcohol consumption.
The aim of this study was to investigate the relationship between MUC6 VNTR polymorphisms and H pylori infection. We were able to confirm that MUC6 VNTR length is highly polymorphic and our data suggest that H pylori infection is more frequent in patients with short MUC6 alleles.
Few other studies investigated the MUC6 VNTR polymorphism. Vinall et al[6] found 11 different allele fragment lengths for MUC6, ranging from 8 to 13.5 kb. This degree of variation is considerably lower than in our study. It is unlikely that this difference in VNTR length variation is inherent to the Southern blotting technique, as we used the same restriction enzyme. However, the aforementioned study used the Centre d’Étude du Polymorphisme Humain (CEPH) series of families in France and these may be much more homogeneous than our study population.
The relationship between H pylori infection and mucin VNTR length has been investigated for another mucin gene, MUC1, and the results were similar to ours. Like other mucins, MUC1 lubricates epithelial structures and constitutes a barrier against acid, proteases and pathogenic organisms. Vinall et al[9] showed that short MUC1 alleles were associated with H pylori-induced gastritis. Therefore, MUC1 and MUC6, although arising from different families of mucins, may be involved in the same mechanism regarding H pylori infection[14].
Our results are also compatible with research focusing on the relationship between mucin allele length and gastric cancer. In a study investigating 157 gastric cancer patients, it was found that short MUC6 alleles were more frequent in patients with gastric cancer than in healthy blood donors[10]. This seems to be in line with our results that short MUC6 alleles are associated with H pylori infection. In fact, because H pylori has been classified as a class I carcinogen, the higher prevalence of H pylori infection among patients with short MUC6 alleles may (partly) explain the higher prevalence of gastric cancer in these patients. Again, the same goes for MUC1 since Carvalho et al[11] stated that short MUC1 alleles were associated with gastric cancer. More research is necessary to determine whether these relationships are independent.
However, other researchers claimed that MUC5AC, and not MUC6, is important in H pylori infection: Van den Brink et al[15] stated that H pylori co-localised with MUC5AC but not with MUC6, and Van de Bovenkamp et al[16] stated that MUC5AC, and not MUC6, was the most important receptor for H pylori. However, in the study by Van den Brink et al, antibodies recognizing the MUC6 precursor rather than mature MUC6 were used. Therefore, it seems plausible that the precursor MUC6 is only found in neck and gland cells, where MUC6 is synthesized. However, the mature MUC6, which is secreted, may be found throughout a much larger area. In fact, Ho et al[17] recently confirmed that the mucin within the glands consisted entirely of MUC6, but they also showed that, although the mucus layer on the gastric surface consisted primarily of MUC5AC, layers of MUC6 were interspersed between the layers of MUC5AC.
Regarding the receptor function of the mucins, MUC5AC is the primary source of Lewis B (Leb), a terminal carbohydrate chain that acts as a ligand for the bacterial adhesion molecule BabA[16,18]. However, although other receptor sites may be present on MUC6[19], mucins may be involved in many other processes besides bacterial binding.
In fact, recently, Kawakubo et al[20] showed that secretions from the glands (consisting of MUC6) may have an antibiotic effect on H pylori, while secretions from the superficial epithelium (primarily consisting of MUC5AC) may have a pro-biotic effect, thereby limiting H pylori infection to the superficial epithelium and protecting the deeper layers of the gastric mucosa. This is consistent with the geographical distribution of H pylori described by Van den Brink et al[15]. The antibiotic effect was mediated by terminal α-1, 4-linked N-acetylglucosamine (α-1,4-GlcNAc) residues, which are present on the variable region of MUC6. Furthermore, the presentation of multiple terminal α-1,4-GlcNAc residues as a cluster may be important for achieving optimal activity. This may explain our finding that shorter MUC6 molecules, which have fewer α-1,4-GlcNAc residues and therefore lower antimicrobial activity, are associated with H pylori infection. More research is necessary to further elucidate the functions of the mucins.
The authors would like to thank Saskia Bergevoet for performing the Southern Blots and the American Gastroenterology Association for the opportunity to present this paper at the Digestive Disease Week (May 2005, Chicago).
S- Editor Wang GP L- Editor Lakatos PL E- Editor Bai SH
| 1. | Kuipers EJ. Helicobacter pylori and the risk and management of associated diseases: gastritis, ulcer disease, atrophic gastritis and gastric cancer. Aliment Pharmacol Ther. 1997;11 Suppl 1:71-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 139] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 2. | Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3126] [Cited by in RCA: 3181] [Article Influence: 132.5] [Reference Citation Analysis (0)] |
| 3. | Brown LM. Helicobacter pylori: epidemiology and routes of transmission. Epidemiol Rev. 2000;22:283-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 620] [Cited by in RCA: 625] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 4. | Ota H, Nakayama J, Momose M, Hayama M, Akamatsu T, Katsuyama T, Graham DY, Genta RM. Helicobacter pylori infection produces reversible glycosylation changes to gastric mucins. Virchows Arch. 1998;433:419-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 66] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Van Klinken BJ, Dekker J, Büller HA, de Bolòs C, Einerhand AW. Biosynthesis of mucins (MUC2-6) along the longitudinal axis of the human gastrointestinal tract. Am J Physiol. 1997;273:G296-G302. [PubMed] |
| 6. | Vinall LE, Hill AS, Pigny P, Pratt WS, Toribara N, Gum JR, Kim YS, Porchet N, Aubert JP, Swallow DM. Variable number tandem repeat polymorphism of the mucin genes located in the complex on 11p15.5. Hum Genet. 1998;102:357-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 66] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Debailleul V, Laine A, Huet G, Mathon P, d'Hooghe MC, Aubert JP, Porchet N. Human mucin genes MUC2, MUC3, MUC4, MUC5AC, MUC5B, and MUC6 express stable and extremely large mRNAs and exhibit a variable length polymorphism. An improved method to analyze large mRNAs. J Biol Chem. 1998;273:881-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 82] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Corfield AP, Myerscough N, Longman R, Sylvester P, Arul S, Pignatelli M. Mucins and mucosal protection in the gastrointestinal tract: new prospects for mucins in the pathology of gastrointestinal disease. Gut. 2000;47:589-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 278] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 9. | Vinall LE, King M, Novelli M, Green CA, Daniels G, Hilkens J, Sarner M, Swallow DM. Altered expression and allelic association of the hypervariable membrane mucin MUC1 in Helicobacter pylori gastritis. Gastroenterology. 2002;123:41-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 92] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Garcia E, Carvalho F, Amorim A, David L. MUC6 gene polymorphism in healthy individuals and in gastric cancer patients from northern Portugal. Cancer Epidemiol Biomarkers Prev. 1997;6:1071-1074. [PubMed] |
| 11. | Carvalho F, Seruca R, David L, Amorim A, Seixas M, Bennett E, Clausen H, Sobrinho-Simoes M. MUC1 gene polymorphism and gastric cancer--an epidemiological study. Glycoconj J. 1997;14:107-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 74] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Toribara NW, Roberton AM, Ho SB, Kuo WL, Gum E, Hicks JW Jr, Gum JR, Byrd JC, Siddiki B, Kim YS. Human gastric mucin. Identification of a unique species by expression cloning. J Biol Chem. 1993;268:5879-5885. [PubMed] |
| 13. | Hegedus O, Ryden J, Rehnberg AS, Nilsson S, Hellstrom PM. Validated accuracy of a novel urea breath test for rapid Helicobacter pylori detection and in-office analysis. Eur J Gastroenterol Hepatol. 2002;14:513-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Dekker J, Rossen JW, Buller HA, Einerhand AW. The MUC family: an obituary. Trends Biochem Sci. 2002;27:126-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 284] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 15. | Van den Brink GR, Tytgat KM, Van der Hulst RW, Van der Loos CM, Einerhand AW, Buller HA, Dekker J. H pylori colocalises with MUC5AC in the human stomach. Gut. 2000;46:601-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 110] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 16. | Van de Bovenkamp JH, Mahdavi J, Korteland-Van Male AM, Buller HA, Einerhand AW, Boren T, Dekker J. The MUC5AC glycoprotein is the primary receptor for Helicobacter pylori in the human stomach. Helicobacter. 2003;8:521-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 108] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 17. | Ho SB, Takamura K, Anway R, Shekels LL, Toribara NW, Ota H. The adherent gastric mucous layer is composed of alternating layers of MUC5AC and MUC6 mucin proteins. Dig Dis Sci. 2004;49:1598-1506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 72] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 18. | Ilver D, Arnqvist A, Ogren J, Frick IM, Kersulyte D, Incecik ET, Berg DE, Covacci A, Engstrand L, Boren T. Helicobacter pylori adhesin binding fucosylated histo-blood group antigens revealed by retagging. Science. 1998;279:373-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 872] [Cited by in RCA: 842] [Article Influence: 31.2] [Reference Citation Analysis (1)] |
| 19. | Nordman H, Davies JR, Lindell G, de Bolós C, Real F, Carlstedt I. Gastric MUC5AC and MUC6 are large oligomeric mucins that differ in size, glycosylation and tissue distribution. Biochem J. 2002;364:191-200. [PubMed] |
| 20. | Kawakubo M, Ito Y, Okimura Y, Kobayashi M, Sakura K, Kasama S, Fukuda MN, Fukuda M, Katsuyama T, Nakayama J. Natural antibiotic function of a human gastric mucin against Helicobacter pylori infection. Science. 2004;305:1003-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 253] [Article Influence: 12.0] [Reference Citation Analysis (1)] |