Published online Sep 28, 2006. doi: 10.3748/wjg.v12.i36.5793
Revised: June 5, 2006
Accepted: June 14, 2006
Published online: September 28, 2006
AIM: To examine human β-defensin-3 (hBD-3) expression in inflamed gastric mucosal tissues or MKN45 gastric cancer cells with or without H pylori infection for better understanding the innate immune response to H pylori.
METHODS: We used reverse transcription-polymerase chain reactions and immunohistochemistry to examine hBD-3 expression in inflamed gastric mucosal tissues or MKN45 gastric cancer cells with or without H pylori. Effects of hBD-3 against H pylori were also evaluated.
RESULTS: The mean mRNA expression of hBD-3 in H pylori-positive specimens was significantly higher than that in H pylori-negative specimens (P = 0.0002, Mann-Whitney). In addition, unlike uninfected samples, 8 of 15 (53.33%) infected mucosal samples expressed hBD-3 protein. H pylori dose-dependently induced mRNA expression of hBD-3 in MKN45 cells, an effect inhibited by adding anti-toll-like receptor (TLR)-4 antibody. HBD-3 protein completely inhibited H pylori growth.
CONCLUSION: Our results suggest that like hBD-2, hBD-3 may be involved in the pathophysiology of H pylori-induced gastritis.
- Citation: Kawauchi K, Yagihashi A, Tsuji N, Uehara N, Furuya D, Kobayashi D, Watanabe N. Human β-defensin-3 induction in H pylori-infected gastric mucosal tissues. World J Gastroenterol 2006; 12(36): 5793-5797
- URL: https://www.wjgnet.com/1007-9327/full/v12/i36/5793.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i36.5793
Defensins are antimicrobial peptide components of the innate immune system. Three subfamilies, α-defensins, β-defensins, and θ-defensins, distinguished according to structural features at the gene and protein levels, have been identified in vertebrates[1-3]. Four human β-defensins (hBD) have recently been characterized in various human epithelial cells. HBD-1 mRNA is expressed constitutively in various epithelial tissues[1-2]. HBD-2 mRNA expression has been detected in epithelial cells of skin, lung, trachea, and urogenital tract; expression can be induced by treatment with tumor necrosis factor (TNF)-α or interleukin (IL)-1, or by exposure to microorganisms[1-9]. Both hBD-1 and hBD-2 show antimicrobial activity, predominantly against Gram-negative bacteria. In skin, tonsil, and trachea, hBD-3 mRNA expression can be induced in epithelial cells by treatment with TNF-α or contact with Pseudomonas aeruginosa[9]. HBD-3 protein shows antimicrobial activity against both Gram-negative and Gram-positive bacteria[9]. Intense hBD-4 mRNA expression has been found in testis and gastric antrum, with lower, presumably constitutive, expression observed in uterus, thyroid gland, lung, kidney, and neutrophils[10]. HBD-4 mRNA expression can be induced by treatment with phorbol 12-myristate 13-acetate (PMA) or contact with P. aeruginosa or Streptococcus pneumoniae in SAEC 6043 small airway epithelial cells, but not by IL-1, IL-6, interferon (IFN)-γ, or TNF-α. HBD-4 shows antimicrobial activity against both Gram-negative and Gram-positive bacteria[10]. However, isolation of natural hBD-4 has not yet been reported.
Recently, induction of hBD-2 mRNA expression by H pylori has been shown in human gastric cancer cell lines (MKN 45 and AGS)[4-8]. Gastric colonization by H pylori, which is Gram negative, is pathogenetically important in gastritis, peptic ulcer, gastric cancer, and gastric mucosa-associated lymphoid tissue lymphoma. However, hBD-3 mRNA expression in gastric mucosal tissues has not been fully characterized[1].
To better understand the innate immune response to H pylori, we determined hBD-3 expression in various gastric mucosal tissues with or without H pylori infection using a semiquantitative TaqMan reverse transcription-polymerase chain reaction (RT-PCR) assay as well as immunohistochemistry. Additionally, the antimicrobial effect of hBD-3 against H pylori was evaluated.
H pylori (ATCC49504) was used for hBD-2 mRNA and hBD-3 mRNA induction. Anti-toll-like receptor (TLR)-4 antibody (Clone: HTA125) and non-immune subclass-matched antibody (IgG2a) were purchased from BD Biosciences Pharmingen. Polyclonal goat antibodies against hBD-2 and hBD-3 were purchased from Santa Cruz Biotechnology.
MKN-45 gastric cancer cells were cultured in RPMI 1640 medium (Bio Whittaker) supplemented with heat-inactivated fetal bovine serum (FBS) (JRH BIOSCIENCES) at 37°C in an humidified atmosphere containing 50 mL/L CO2. Induction of hBD-2 mRNA and hBD-3 mRNA was carried out as described previously[7,8]. Briefly, 106 MKN45 cells were seeded into dishes 60 mm in diameter and incubated for 12 h. Culture medium was replaced with 2 mL of fresh RPMI 1640 medium without FBS. Bacterial suspensions (100 μL; 0 to 109 CFU/mL in RPMI 1640 medium) were added to the dishes, and incubation was continued for various time periods.
Samples of non-cancerous mucosa with or without chronic gastritis were obtained from 25 patients with previously untreated gastric cancer following surgery at Sapporo Medical University Hospital. Informed consent was obtained from all patients. After tissue removal, all samples were immediately frozen and fixed in 100 mL/L formalin.
Sections were Giemsa-stained, and the rapid urease test (CLO test, Tri-Med Specialties Inc) was performed with fresh samples taken from the prepyloric antrum, greater curvature of the corpus, and fundus[12]. H pylori infection was defined as positive when H pylori was detected and/or the CLO test was positive.
ISOGEN (Nippon Gene) was used to extract total RNA from cells or tissues, and this extract was assayed for RNA with the GeneQuant DNA/RNA calculator (Amersham Pharmacia Biotech). For quantitative RT-PCR, fluorescent hybridization probes, TaqMan PCR Core Reagents kit with AmpliTaq Gold (Perkin-Elmer Applied Biosystems) were used with the ABI Prism 7700 Sequence Detection System (Perkin- Elmer Applied Biosystems). Expression of hBD mRNA was quantified as previously described[8,13]. Primers and TaqMan probe for hBD-2 mRNA were as follows: 5’-TGGTGGTATAGGCGATCCTGTT-3’ (forward) and 5’-GGAGACCACAGGTGCCAATTT-3’ (reverse); 5’-CCATATGTCATCCAGTTCTT-3’ (TaqMan probe). Primers and TaqMan probe for hBD-3 mRNA were as follows. 5’-AGTGACCAAGCACACCTTTTCA-3’ (forward) and 5’-CCAAAAACAGGAAGAGCAAAGC-3’ (reverse); 5’-TATGAGGATCCATTATCTTCTGTT-3’ (TaqMan probe). Aliquots of 25 ng of total RNA from samples was used for one-step RT-PCR. Conditions of one-step RT-PCR were as follows: 30 min at 48°C (stage 1, reverse transcription), 10 min at 95°C (stage 2, RT inactivation and AmpliTaq Gold activation), and then 40 cycles of amplification for 15 s at 95°C and 1 min at 60°C (stage 3, PCR). Data were normalized as the ratio of hBD-2 optical densities relative to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and were represented as arbitrary units.
Formalin-fixed, paraffin-embedded tissue sections were stained with polyclonal goat antibodies against hBD-2, hBD-3 or non-immune goat serum using an indirect immunoperoxidase technique.
To evaluate the antimicrobial effects of hBD-2 and hBD-3 on H pylori, 25 μL of 4 × 106 CFU/mL H pylori strain (ATCC49504) was cultured on HP agar (Eikenkagaku) after a 1-h pre-incubation at 37°C in the presence or absence of hBD-2 protein and hBD-3 protein (Peptide Institute). To determine the CFU number, the pre-incubation mixture was immediately diluted 100-fold with culture medium, and samples were cultured in triplicate. Viable cells (CFU/mL) were counted after 3 d in culture at 37°C.
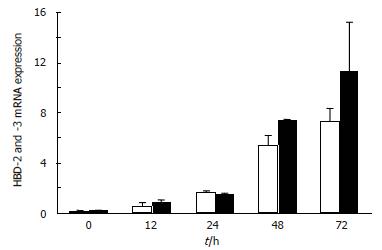
To clarify the effects of H pylori on hBD-2 mRNA and hBD-3 mRNA expression, according to TaqMan RT-PCR, MKN45 cells first were incubated for 0 to 72 h with H pylori. HBD-2 mRNA and hBD-3 mRNA expression was detected in MKN45 cells 12 h after initiating incubation with H pylori, and then increased gradually (Figure 1).
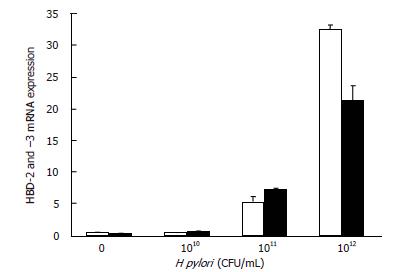
To determine a suitable number of H pylori bacteria for induction of hBD-2 mRNA and hBD-3 mRNA expression, 100 μL of 0 to 109 CFU/mL H pylori was incubated with MKN45 cells for 48 h, representing multiple inoculum sizes. HBD-2 mRNA and hBD-3 mRNA expression was up-regulated in a manner dependent on bacterial number (Figure 2), an effect first detectable at 107 CFU/mL.
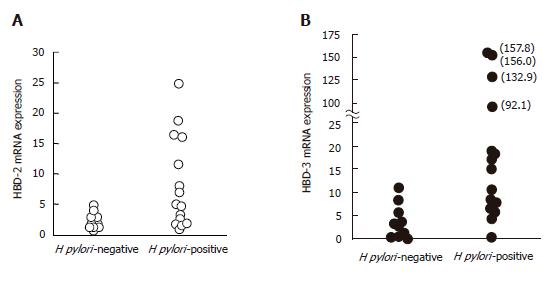
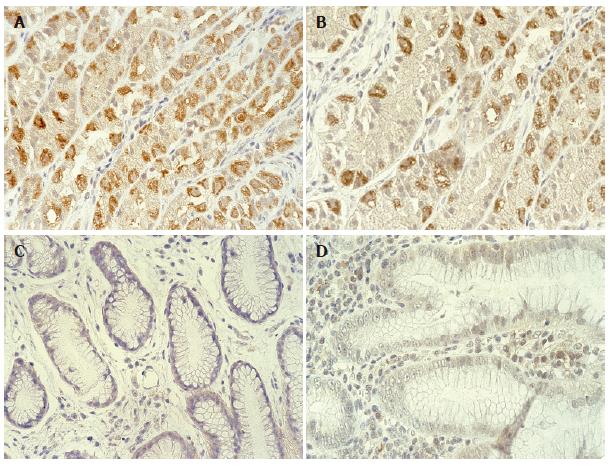
To evaluate the effect of H pylori colonization in gastric tissues on hBD-2 and hBD-3 expression, mucosal samples showing gastritis from 15 H pylori-positive and 10 H pylori-negative patients were assessed by TaqMan RT-PCR analysis and immunostaining. Mean mRNA expression of hBD-2 (8.5) and hBD-3 (45.0) in H pylori-positive specimens was significantly higher than that in hBD-2 (1.6) and hBD-3 (3.9) in H pylori-negative specimens, respectively (Figure 3A and B; P = 0.002, Mann-Whitney). However, levels of hBD-3 mRNA expression did not correlate with those of hBD-2 mRNA expression in H pylori-positive specimens. In addition, 7 and 8 of 15 H pylori-positive patients with gastritis showed hBD-2 and hBD-3 protein expression in their mucosa, respectively, while none of the H pylori-negative patients showed hBD-2 and hBD-3 protein expression (Figure 4A-D).
To determine whether hBD-2 and hBD-3 mRNA expression in MKN45 cells was induced via TLR-4, MKN45 cells were incubated for 40 h with 100 μL of 109 CFU/mL of H pylori in the presence or absence of anti-TLR-4 antibody (100 μg/mL) and control antibody (100 μg/mL). Expression of hBD-2 and hBD-3 mRNA in H pylori-exposed MKN45 cells treated with anti-TLR-4 antibody was reduced to 55.7% and 64.1% of the expression in untreated MKN45 cells, respectively.
To evaluate the antimicrobial effects of hBD-2 and hBD-3 on H pylori, H pylori was cultured on HP agar for 4 d after a 1-h pre-incubation in the presence or absence of chemically synthesized hBD-2 and hBD-3. At concentrations of 50 μg/mL or more, hBD-2 and hBD-3 inhibited growth of H pylori (Figure 5A and B).
Using TaqMan RT-PCR for hBD-3 mRNA and immunostaining for hBD-3 protein, we demonstrated, probably for the first time, that like hBD-2, hBD-3 is frequently expressed in gastric mucosa with H pylori infection showing gastritis, but not in inflamed mucosa without H pylori infection. The amount of hBD-3 mRNA expression in H pylori-positive specimens was 11.5 times higher than in H pylori-negative specimens. In addition, hBD-3 was induced by contact of MKN45 cells with H pylori. Recent reports indicated that hBD-3 mRNA expression was induced in primary cultures of tracheal epithelial cells by pro-inflammatory cytokines, such as TNF-α, and in the MKN7 gastric cancer cell line by contact with H pylori. Considered together with recent reports, our results implied that contact of gastric epithelial cells with H pylori as well as amounts of pro-inflammatory cytokines are important for induction of hBD-3 mRNA expression.
At 10-5 mol/L, chemically synthesized hBD-2 (30 μg/mL) was reported to completely inhibit growth of H pylori, while recombinant hBD-3 began to inhibit growth of H pylori at a concentration of 10-7 mol/L[5,11]. In the present study, we demonstrated that synthesized hBD-3 as well as hBD-2 inhibited H pylori growth at concentrations of 50 μg/mL or more.
Isomoto et al[14] detected activated nuclear factor (NF)-κB in epithelial cells in gastric mucosa from patients with H pylori-associated gastritis. Diamond et al[15] reported that expression of hBD-3 was regulated by NF-κB-independent mechanisms, that remain to be characterized. Su et al[16] reported that H pylori up-regulated TLR-4 expression in two gastric cancer cell lines (AGS and MKN45). These reports suggest that H pylori may induce hBD-3 mRNA expression via direct or indirect activation of NF-κB or other mechanisms. Pathogen-associated molecular patterns in H pylori and pattern recognition receptors in gastric epithelial cells will require further study. In the present investigation, anti-TLR-4 antibody inhibited, but not completely, the expression of hBD-3 mRNA in MKN45 cells induced by contact with H pylori. This suggests that not only H pylori binding to TLR-4, but also other mechanisms may exist for the induction of hBD-3 mRNA in gastric epithelial cells by H pylori infection. In addition, in the present study, levels of hBD-3 mRNA expression did not correlate with those of hBD-2 mRNA expression in H pylori-positive specimens. This also suggests that the expression of hBD-3 and hBD-2 mRNA in gastric epithelial cells is likely to be regulated differently.
In conclusion, expression of hBD-3 increases in response to H pylori infection with a bacterial effect. Further study is needed to detect the role and regulating mechanism of hBD-3.
S- Editor Pan BR L- Editor Kumar M E- Editor Bai SH
| 1. | Selsted ME, Ouellette AJ. Mammalian defensins in the antimicrobial immune response. Nat Immunol. 2005;6:551-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 874] [Cited by in RCA: 886] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 2. | Schneider JJ, Unholzer A, Schaller M, Schäfer-Korting M, Korting HC. Human defensins. J Mol Med (Berl). 2005;83:587-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 255] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 3. | Harder J, Bartels J, Christophers E, Schröder JM. A peptide antibiotic from human skin. Nature. 1997;387:861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1015] [Cited by in RCA: 972] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 4. | Wada A, Ogushi K, Kimura T, Hojo H, Mori N, Suzuki S, Kumatori A, Se M, Nakahara Y, Nakamura M. Helicobacter pylori-mediated transcriptional regulation of the human beta-defensin 2 gene requires NF-kappaB. Cell Microbiol. 2001;3:115-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 69] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 5. | Hamanaka Y, Nakashima M, Wada A, Ito M, Kurazono H, Hojo H, Nakahara Y, Kohno S, Hirayama T, Sekine I. Expression of human beta-defensin 2 (hBD-2) in Helicobacter pylori induced gastritis: antibacterial effect of hBD-2 against Helicobacter pylori. Gut. 2001;49:481-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 88] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 6. | O'Neil DA, Cole SP, Martin-Porter E, Housley MP, Liu L, Ganz T, Kagnoff MF. Regulation of human beta-defensins by gastric epithelial cells in response to infection with Helicobacter pylori or stimulation with interleukin-1. Infect Immun. 2000;68:5412-5415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 89] [Article Influence: 3.6] [Reference Citation Analysis (1)] |
| 7. | Wada A, Mori N, Oishi K, Hojo H, Nakahara Y, Hamanaka Y, Nagashima M, Sekine I, Ogushi K, Niidome T. Induction of human beta-defensin-2 mRNA expression by Helicobacter pylori in human gastric cell line MKN45 cells on cag pathogenicity island. Biochem Biophys Res Commun. 1999;263:770-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Uehara N, Yagihashi A, Kondoh K, Tsuji N, Fujita T, Hamada H, Watanabe N. Human beta-defensin-2 induction in Helicobacter pylori-infected gastric mucosal tissues: antimicrobial effect of overexpression. J Med Microbiol. 2003;52:41-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Harder J, Bartels J, Christophers E, Schroder JM. Isolation and characterization of human beta -defensin-3, a novel human inducible peptide antibiotic. J Biol Chem. 2001;276:5707-5713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1017] [Cited by in RCA: 1004] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 10. | García JR, Krause A, Schulz S, Rodríguez-Jiménez FJ, Klüver E, Adermann K, Forssmann U, Frimpong-Boateng A, Bals R, Forssmann WG. Human beta-defensin 4: a novel inducible peptide with a specific salt-sensitive spectrum of antimicrobial activity. FASEB J. 2001;15:1819-1821. [PubMed] |
| 11. | George JT, Boughan PK, Karageorgiou H, Bajaj-Elliott M. Host anti-microbial response to Helicobacter pylori infection. Mol Immunol. 2003;40:451-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Marshall BJ, Warren JR, Francis GJ, Langton SR, Goodwin CS, Blincow ED. Rapid urease test in the management of Campylobacter pyloridis-associated gastritis. Am J Gastroenterol. 1987;82:200-210. [PubMed] |
| 13. | Yajima T, Yagihashi A, Kameshima H, Kobayashi D, Furuya D, Hirata K, Watanabe N. Quantitative reverse transcription-PCR assay of the RNA component of human telomerase using the TaqMan fluorogenic detection system. Clin Chem. 1998;44:2441-2445. [PubMed] |
| 14. | Isomoto H, Mizuta Y, Miyazaki M, Takeshima F, Omagari K, Murase K, Nishiyama T, Inoue K, Murata I, Kohno S. Implication of NF-kappaB in Helicobacter pylori-associated gastritis. Am J Gastroenterol. 2000;95:2768-2776. [PubMed] |
| 15. | Diamond G, Bevins CL. beta-Defensins: endogenous antibiotics of the innate host defense response. Clin Immunol Immunopathol. 1998;88:221-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 102] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 16. | Su B, Ceponis PJ, Lebel S, Huynh H, Sherman PM. Helicobacter pylori activates Toll-like receptor 4 expression in gastrointestinal epithelial cells. Infect Immun. 2003;71:3496-3502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 117] [Article Influence: 5.3] [Reference Citation Analysis (0)] |