CARDITIS AND INTESTINAL METAPLASIA AT THE GASTRO-ESOPHAGEAL JUNCTION
The incidence of adenocarcinoma of the cardia of the stomach and adjacent gastro-oesophageal (GO) junction has been rising over the past 25 years, especially in Western countries[1]. In recent years, a similar trend has been reported in Japan, although GO junction adenocarcinoma is still rare[2]. We recently reported that this type of cancer occurs in subjects with preserved gastric acid secretion and is not associated with H pylori infection[3]. Although gastric acid, pepsin, and bile have a deleterious effect on the epithelium at that site, the carcinogen for this type of cancer remains unknown. A sequence of histological events in which chronic inflammation progresses to metaplasia, dysplasia and, finally, carcinoma has been documented in distal gastric cancer, and is associated with H pylori infection in most cases. The sequence is also well defined for esophageal adenocarcinoma through the formation of intestinal metaplasia (IM) in the distal esophagus (Barrett’s esophagus). Although such a process has not been established in the cardia, a similar sequence of events would occur at that site as IM at the squamo-columnar junction which was shown to have a malignant potential similar to that of Barrett’s esophagus[4,5].
During the last decade, inflammation (carditis) and IM localized to the immediate vicinity of the squamo-columnar junction have received much attention in relation to the rising incidence of cancer at this site. These histological findings are reported to be highly prevalent (38%-79% for carditis[6-10], 10%-36% for IM[7,11-15]) even among healthy subjects. Of these two histological parameters, the junctional IM is usually associated with carditis[7,15], and hence the IM could be assumed to be a consequence of chronic inflammation at the cardia as in more distal areas of the stomach[18]. There are two distinct patterns of carditis[6,7,9,10]. One is associated with H pylori infection and is characterized by inflammation in other sites of the stomach. The other, which is unassociated with H pylori infection, comprises inflammation confined to the cardiac mucosa and is seen in patients with otherwise healthy stomachs. There are also differences in the grade and activity of the inflammatory infiltrate involving the columnar mucosa at the squamo-columnar junction, that is, patients with H pylori-associated carditis have more severe inflammation than patients with healthy stomachs and neutrophil infiltration is uncommon in the latter group[6,7,10,17]. Although carditis in H pylori-negative subjects is sometimes accompanied by the presence of gastro-oesophageal reflux disease[6-10,16,17], its cause remains obscure and it may well represent the histological response to many different types of insult. Considering the milder inflammation usually seen in the absence of neutrophil infiltration, chemical insult can be a probable candidate as a cause of the carditis.
LUMINAL NITRIC OXIDE GENERATION AT THE GASTRO-OESOPHAGEAL JUNCTION
Recently, the hypothesis that a high concentration of luminal nitric oxide (NO) at the human GO junction after nitrate ingestion may be relevant to various diseases at that site was made by a group of researchers in Glasgow[19,20]. Since then, that group and the present authors have been doing further research on this issue[21-24].
NO is an important radical that mediates a wide range of physiologic and pathologic events. It is generated at low concentrations by the enzyme constitutive nitric oxide synthase (cNOS) to modulate neuromuscular and vascular functions. Higher concentrations are generated by the inducible form of the enzyme as a part of the immune and inflammatory response. Sustained generation of NO by inducible NO synthase (iNOS) has been implicated in the aetiology of the mutagenesis and neoplasia related to chronic inflammation.
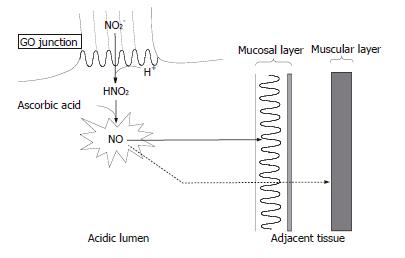
The highest concentrations of NO occurring in the body are not the result of enzymatic synthesis but rather by chemical reactions within the lumen of the stomach, especially in the most proximal area. A previous study reported that high, potentially mutagenic concentrations of NO are generated luminally at the GO junction through the entero-salivary re-circulation of dietary nitrate in humans[19]. This is explained as follows. Ingested nitrate as an ingredient of food is absorbed from the small intestine, and 25% of this is re-secreted into the mouth by the salivary glands. Bacteria on the dorsum of the tongue then reduce about 30% of this nitrate to nitrite. When salivary nitrite enters the stomach, the combination of the acidity and the ascorbic acid content of the gastric juice converts the nitrite to NO. Since this reaction between nitrite and ascorbic acid at an acidic pH is very rapid[21], the intraluminal concentration of NO generated by the reaction is maximal at the GO junction and cardia, where the nitrite in saliva first encounters gastric acid. Indeed, this was confirmed by the afore-mentioned study in healthy volunteers which reported that substantial amounts of NO are generated following nitrate ingestion at these anatomical locations, in some case in excess of 50 μmol/L[19].
The entero-salivary re-circulation of dietary nitrate is sustained for several hours, during which period the adjacent epithelium of the GO junction is exposed to abundant amounts of NO generated in the lumen. Membranes in the tissues are not barriers to the diffusion of NO because of its gaseous and lipophilic properties. Since NO is known to have dual effects within the tissue, i. e. cytoprotective and cytotoxic depending on the gas level, determination of the NO level is required to evaluate its function in the tissue. We recently developed a rat animal model in which nitrite and acidified ascorbic acid were administered separately so that the generation of NO from nitrite would be maximal at the GO junction where the reactants first meet[24]. In that model, we demonstrated by means of electron paramagnetic resonance spectroscopy with an exogenously supplied NO trapping agent that the NO generated luminally diffused into adjacent tissues to a substantial degree and at a level comparable to that of iNOS derived-NO production and altered the integrity of the surrounding epithelium. As we applied a concentration of nitrite that has been observed in human saliva after the ingestion of a high nitrate meal, this finding may be applicable to the GO junction in humans[24] (Figure 1).
Figure 1 Nitric oxide chemistry at human gastro-oesophageal junction.
Nitrite (NO2-) in swallowed saliva is converted to nitric oxide (NO) promptly at the gastro-oesophageal (GO) junction where encountering gastric acid containing ascorbic acid. NO thus formed diffuses into the adjacent tissue of the GO junction because of its gaseous and lipophilic properties. The majority of the NO arising from the lumen will be exhausted within the superficial mucosal layer by reacting with surrounding molecules while a small portion of the NO can reach the inner muscular layer. The luminal generation of NO is sustained for several hours after nitrate ingestion, during which period the GO junction is exposed to abundant amounts of NO.
Briefly, a high level of salivary nitrite is sustained over several hours after the ingestion of a high nitrate meal and the nitrite in swallowed saliva is rapidly converted to NO at the GO junction. Then, a substantial amount of NO eventually diffuses from the lumen into the adjacent tissue. Therefore, the human GO junction is likely to be a region of high nitrosative stress. Considering the life-time exposure of the GO junction to a high level of NO, this may account for the high prevalence of inflammation and IM at that site. Assuming the metaplasia-dysplasia-adenocarcinoma sequence at the GO junction, NO may eventually lead to the development of carcinoma at that site. In addition to being mediated through such a sequence, a high concentration of NO can directly exert a mutagenic and carcinogenic effect through the formation of higher oxides of nitrogen such as N2O3, which can damage DNA directly via deamination of bases and indirectly by forming N-nitrosocompounds. N2O3 is also known to inactivate DNA repair enzymes such as O6-alkylguanine DNA alkyltransferase[25-27].
POSSIBLE IMPLICATIONS OF NITRIC OXIDE AT THE GASTRO-OESOPHAGEAL JUNCTION
In this chemical reaction occurring at the GO junction, the presence of sufficient gastric acid is essential, and this condition is ensured only at the GO junction and the cardia after eating, where the acidic gastric juice largely escapes the buffering effect of food, which decreases the acidity of the gastric juice in the rest of the stomach[28]. The necessity of gastric acid for luminal NO generation is consistent with our recent clinical study which found that the preservation of gastric acid is necessary for the development of cancer at the GO junction[3]. In addition, a recent study has demonstrated that the site of NO generation shifted orally to the distal esophagus in cases with gastro-oesophageal reflux[23]. Hence, the NO thus generated in the distal esophagus may also be relevant to the formation of Barrett’s oesophagus and oesophageal adenocarcinoma.
Interestingly, although the majority of NO arising from the lumen would become exhausted within the superficial epithelial layer in our rat model, a small but significant amount of NO was detected even in the inner muscular layer of the GO junction, especially when a high concentration of nitrite was administered[24]. This suggests that a part of the nitric oxide arising from the lumen could escape from autoxidation occurring within the superficial layer and reach the inner tissue. It is well known that NO endogenously derived from cNOS localized to non-adrenergic, non-cholinergic nerves mediates the relaxation of the smooth muscle cells, including those of the lower esophageal sphincter[29]. An in vitro study using muscle strips from opossum lower esophageal sphincter demonstrated that a tiny amount of NO (in the range of nmol/L) was sufficient to induce relaxation of the muscle[30]. Therefore, the amount of NO formed in the lumen at the human GO junction may be sufficient to penetrate the epithelium and then affect the inner smooth muscle cell of the lower esophageal sphincter, leading to relaxation of the muscle and reflux of the acidic gastric content into the oesophagus. Actually, a recent report demonstrated in humans that NO generated luminally at the GO junction did affect the lower esophageal sphincter, leading to a significant increase in the transient relaxation of the lower esophageal sphincter[31]. Further studies are required to clarify to what extent this phenomenon is responsible for the pathogenesis of ordinary gastro-oesophageal reflux disease in humans. However, it may be related at least to the symptoms of heartburn that are known to be invoked after the ingestion of certain foods[32,33] (Figure 1).
Needless to say, NO is known to have some beneficial effects on the stomach as well. It has been shown that intragastric topical application or parental administration of nitric oxide-releasing substances protects the gastric mucosa from damage induced by injurious agents, suggesting that NO plays an important role in the protection of the gastric mucosa[34-36]. However, this protective effect is dose-sensitive; as some studies have reported that high doses of exogenous NO can exacerbate gastric mucosal injury[37,38]. Thus, a high concentration of local NO at the gastric cardia may be involved in the pathology of mucosal injury at that site as a potential cytotoxic mediator. On the other hand, a relatively low concentration of nitric oxide as seen in the distal stomach may function as a protective mediator to maintain the gastric mucosal integrity. Similarly, ascorbic acid, which is secreted in the healthy human stomach, could show even an undesirable effect on the GO junction by the rapid conversion of salivary nitrite to NO at that site[21,22], although the vitamin is well known to have beneficial effects as an antioxidant in the remaining stomach.
FUTURE STUDIES ON THE RELEVANCE OF NITRIC OXIDE TO THE VARIOUS DISEASES AT THE GASTRO-OESOPHAGEAL JUNCTION
Further studies to investigate the effect of the diffused NO on the cell biology in the tissue are required to clarify the pathologic relevance of NO to the various histological events occurring at the human GO junction. Accordingly, the characteristic features of NO, for example, the gas is generated abundantly by the unique chemical reaction in the acidic lumen, need to be considered with reference to other examples of pathological circumstances involving NO such as models of inflammation in humans. First, in inflammation, not only NO but other radicals such as superoxides are also generated from inflammatory cells and then the reaction products with NO (for example, peroxynitrite), not NO itself, may be exerting deleterious effects on the tissue[39]. In contrast, the effect of the diffused NO on the tissue at the GO junction should be mainly an NO-mediated process, at least at the early stage, although once the inflammatory process is initiated by the diffused NO other radicals are also formed. Second, since once the NO is diffused from the acidic lumen into the tissue at neutral pH it will be irreversibly metabolized to nitrite and nitrate, it is the very surface of the epithelium that is exposed to the highest concentration of NO, in contrast with examples of inflammation in which the immediate vicinity of inflammatory cells expressing iNOS would be exposed to the highest levels of endogenous NO[39]. Hence, the tight junction of the surface epithelium at the GO junction is likely to be the principal target of the NO arising from the lumen[40,41]. Third, as opposed to the inflammation that occurs only under specific circumstances, the exposure of the human GO junction to localized luminal NO occurs as long as the stomach is healthy and has sufficient acid secretion. In fact, it has been demonstrated that, after the ingestion of nitrate, the luminal NO concentration was maximal at the GO junction in 73% of subjects, although the gas level varied among individuals[19]. Thus, it appears reasonable that the locally generated NO is the cause of inflammation and IM at the cardia, both of which are frequently observed even in healthy subjects as previously mentioned. However, since neoplasia develops only in a small number of people, individual susceptibility to NO toxicity such as from mutations in cancer-related genes (for example p53) may be important in determining its ultimate contribution to carcinogenesis at the GO junction[42].
In conclusion, a cytotoxic concentration of NO is generated luminally at the human GO junction and the gas diffuses into the adjacent tissue. Although gastric acid, pepsin, and bile acid have been intensively investigated as a cause of adenocarcinoma at the GO junction and the distal esophagus, NO and the related nitrosative stress should also be examined.
S- Editor Liu Y L- Editor Alpini GD E- Editor Bi L