Published online Sep 14, 2006. doi: 10.3748/wjg.v12.i34.5557
Revised: May 28, 2006
Accepted: June 14, 2006
Published online: September 14, 2006
AIM: To investigate the effect of serum derived from rats treated with electroacupuncture at stomach meridian acupoints on the expression of epidermal growth factor receptor (EGFR) gene in gastric mucosal cells.
METHODS: The stress-induced gastric mucosal injury in rat model was established by water-immersion and restrained stress methods. 52 rats were randomly divided into: normal group (n = 8), model group (n = 8), model serum group (n = 12), stomach serum group (n = 12), and gallbladder serum group (n = 12). The gastric mucosal cells were separated by pronase-EDTA digestion method and incubated with serum. The EGFR gene expression in gastric mucosal cells was detected by reverse transcription-polymerase chain reaction (RT-PCR) method.
RESULTS: Compared with normal group (0.6860 ± 0.0594), the serum derived from rats of the stomach group (1.2272 ± 0.0813, P = 0.00 < 0.01) and gallbladder group (0.9640 ± 0.0387, P = 0.00 < 0.01) had a tendency to enhance the EGFR gene expression in gastric mucosal cells. Such tendency existed in the model group (0.7104 ± 0.0457) but with no significant difference (P = 0.495 > 0.05) and in model serum group (0.8516 ± 0.0409) with an extremely obvious difference (P = 0.001 < 0.01). Furthermore, the EGFR gene expression in stomach serum group was significantly higher than that in gallbladder serum group (P = 0.00 < 0.01).
CONCLUSION: The present study shows that serum derived from rats treated with electroacupuncture at stomach meridian acupoints can distinctly increase the EGFR gene expression of gastric mucosal cells. Therefore, there is certain meridian specificity in the serum, which could provide a proof for the TCM theory “particular relation between meridian and internal organ”.
- Citation: Yang ZB, Yan J, Zou XP, Yi SX, Chang XR, Lin YP, Li XP. Enhanced expression of epidermal growth factor receptor gene in gastric mucosal cells by the serum derived from rats treated with electroacupuncture at stomach meridian acupoints. World J Gastroenterol 2006; 12(34): 5557-5561
- URL: https://www.wjgnet.com/1007-9327/full/v12/i34/5557.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i34.5557
Gastric mucosal damage is a common pathological reaction in the diseases of the digestive system. The acupuncture and moxibustion are very effective cure for this damage[1,2]. Previous experimental studies demonstrated that epidermal growth factor (EGF) and transforming growth factor-α (TGF-α) were the most important peptides for the repair of the gastric mucosal injury[3]. Acupuncture at gastric meridian acupoint could alter gastric motility and secretion and also the content of gastrin, substance P, EGF and TGF-α in serum and gastric mucosa[4,5]. Recent research indicated that EGFR was closely related to the healing of impaired gastric mucosa, which was of great importance to the gastric mucosal protection and repair after damage[6]. The EGFR belongs to the family of trans-membrane tyrosine protein kinase (TPK). Activation of EGFR stimulates cell proliferation, differentiation, adhesion, and migration[7,8]. The aim of this study was to examine the effect of serum derived from rats treated with electroacupuncture at stomach meridian acupoints on the expression of EGFR gene in gastric mucosal cells. This would hopefully clarify the humoral mechanism of acupuncture effect on gastric mucosal cells and the essential correlation of the meridian acupoints and internal organs.
Pronase and dithiothreitol (DTT) were purchased from MERK. Bovine serum albumin (BSA) was obtained from Biosharp. Percoll was purchased from Pharmacia, Dulbecco’s Modified Eagle Medium (DMEM) from Hyclone, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) from Biosource, Trizol reagent was obtained from Invitrogen. AMV reverse transcriptase, ribonuclease inhibitor (RNasin), dNTPs, Taq DNA polymerase, 100 bp DNA ladder, diethylpyrocarbonate (DEPC), oligodT18 primer, and gelose were purchased from Promega. Tyrosine kinase inhibitor (PD153035) was purchased from Calbiochem. EGFR and the internal control, glyseraldehyde-3-phosphate dehydrogenase (GAPDH) were obtained from Invitrogen. All other reagents were analytically pure.
Water-immersion and restrained stress methods were adopted[9]. Before modelling, the experimental rats were fasted for 24 h and had free access to water only. Rats were fixed on boards and were immersed vertically in a homeostatic bath at 23 ± 1°C for 10 h, with the liquid surface up to the level of the xiphoid process of the sternum.
Sprague-Dawley (SD) male and female rats, with an average weight of 200 ± 30 gm, were supplied by the Experimental Animal Center at Hunan Agriculture University (Permission number: 20030316) 2 wk before the experiment. During this period, they had access to Purina rat chow and water. Animals were fasted overnight before the experiments. Fifty-two rats were randomly divided into normal group, model group, model serum group, stomach serum group and gallbladder serum group. 8 rats were in the normal group and model group. Each of the model serum group, stomach serum group and gallbladder serum group included 12 rats.
Four rats of each of the model serum group, stomach serum group, and gallbladder serum group were selected at random for deriving serum, and the remaining 8 rats were used for isolating gastric mucosal cells. Acupoints location was defined by reference of rat-acupoint-atlas and analogy to human body[10]. According to the induction stated above, three pairs of acupoints consisting of Sibai (ST 2), Liangmen (ST 21), and Zusanli (ST36) in the stomach Meridian, were designed, which represent acupoints of different level (head, trunk, and limb). Also, 3 pairs of acupoints of the gallbladder Meridian in the same horizontal level were selected: Yangbai (GB 14), Riyue (GB24), and Yanglingquan (GB 34).
Pairs of stainless-steel needles of 0.25 mm in diameter were inserted into the acupoints stated above in experimental rats. The needles were connected to the output of an electronic pulse generator, a medical electroacupuncture stimulator (Model G6805-1, made by Shanghai Medical Electro-apparatus Factory, China), which achieves intermittent-and-irregular wave (intermittent wave: 4 Hz, irregular wave: 20 Hz), constant time of 30 min per day, ten days, while there was a light vibration in the lower limbs of rats.
Animals were fasted overnight before the experiments. All experiments were performed using freshly isolated gastric mucosal cells. The contents of the stomach were washed out with phosphate-buffered saline (PBS). The stomach was then ligated at the base of the forestomach and the proximal end of the antrum to obtain mucosal cells primarily from the oxyntic region. After being transformed into inside-out gastric bags, they were filled with 2.5 mL of 1 mg/mL pronase solution in buffer A (0.5 mmol/L NaH2PO4, 1.0 mmol/L Na2HPO4, 20 mmol/L NaHCO3, 80 mmol/L NaCl, 5.0 mmol/L KCl, 50 mmol/L HEPES, 11 mmol/L glucose, 0.02 mmol/L BSA, 2 mmol/L EDTA, pH 7.4). The filled gastric bags were incubated in pronase-free buffer A at 37°C for 30 min. The gastric bags were then transferred into buffer B (0.5 mmol/L NaH2PO4, 1.0 mmol/L Na2HPO4, 20 mmol/L NaHCO3, 80 mmol/L NaCl, 5.0 mmol/L KCl, 50 mmol/L HEPES, 11 mmol/L glucose, 0.01 mmol/L BSA, 1 mmol/L CaCl, 1.5 mmol/L MgCl, pH7.4) and gently agitated by a magnetic stirrer at room temperature for 1h. The gastric mucosal cells dispersed in buffer B were collected by centrifuging at 3000 rpm for 5 min and subsequently re-suspended in serum-free DMEM[11,12].
The blood was sampled from carotid artery after rats were treated according to the requirement of experimental procedures. Then the blood was transferred into centrifuge tubes and placed steadily for 2 h at 37°C. Tubes were centrifuged at 2500 rpm for 10 min. The serum was carefully sucked and frozen at -20°C. The gastric mucosal cells were incubated with 100 mL/L serum at 37°C for 30 min in the experiment[13,14].
Following the treatment stated above, gastric mucosal cells obtained from each rat were collected in Eppendorf tubes and kept in the -80°C. Eight samples from each group were selected randomly for RNA extraction. Total RNA was isolated from samples of gastric mucosal cells by using a guanidium isothiocyanate/phenol chloroform single step extraction kit from Stratagene. Total RNA was precipitated in ethanol and resuspended in sterile RNAase-free water for storage at -80°C untill use. Total RNA was quantified spectrometrically at 260 nm, and the quality of isolated RNA was analyzed on agarose gels under standard conditions.
Total RNA (10 μL, about 0.5 μg/sample) was reverse transcribed (RT) using oligo (dT) 18 primers 1 μL, 5 × RT-buffer 4 μL, dNTPs (10 mmol/L) 1 μL, RNasin (20 MU/μL) 0.5 μL, M-MULV reverse transcriptase (200 MU/μL) 1 μL, and DEPC-treated water 2.5 μL in a 20 μL reverse transcription reaction system. The reaction was performed at 42°C for 30-60 min so that target mRNA was transcribed into cDNA. The tubes were cooled and centrifuged for several seconds.
An aliquot of the RT product of each sample (1/20 of the total volume) was used in the PCR amplification reactions for EGFR and GAPDH. The PCR reaction contained 4 μL cDNA, 10 × PCR buffer 5 μL, dNTPS (10 mmol/L) 1 μL, oligonucleotide primers sense/antisense (10 mmol/L) 1 μL (primer sequences are stated below), Taqase 1 μL, ddH2O 32 μL in a total volume of 50 μL. Reaction mixtures were incubated for predenaturation at 94°C for 2 min, followed by 38 cycles for EGFR (denaturation at 94°C for 30 s, annealing at 53°C for 1 min, and extension at 72°C for 1 min) and 25 cycles for GAPDH (denaturation at 94°C for 30 s, annealing at 56°C for 30 s, and extension at 72°C for 30 s), and a final extension at 72°C for 5 min.
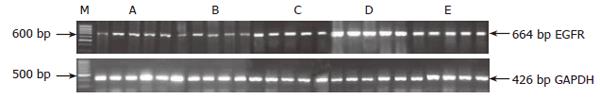
To use of the relatively quantitative method to measure EGFR gene expression, rat GAPDH was selected as internal control substance. The primer sequences and sizes of amplification products are as shown in Table 1. Five microliter PCR products were analyzed on 10 g/L agarose gel containing ethidiumbromide with TBE buffer at 80 V for 40 min and photographed under UV illumination. The band intensities were quantified by densitometry. EGFR and GAPDH PCR products were, respectively, 664 and 426 base pairs (Table 1). EGFR and GAPDH were determined by computer-assisted densitometric scanning. Signals were quantified by density analysis of the digital images using Eagle Eye II image software (Stratagene) and EGFR/GAPDH quotient indicated the relative expression of EGFR. Experiments were performed in triplicate.
| EGFR | Forward primer: | 664 bp |
| 5'-AGT GGT CCT TGG AAA CTT GG-3' | ||
| Reverse primer: | ||
| 5'- GTT GAC ATC CAT CTG GTA CG-3' | ||
| GADPH | Forward primer: | 426 bp |
| 5'-TGC TGA GTA TGT CGT GGA GTC -3' | ||
| Reverse primer: | ||
| 5'-AAG GCC ATG CCA GTG AGC TTC -3' |
The data for each group were expressed as mean ± SD. Comparison between groups was assessed using one-way analysis of variance (ANOVA). Differences were considered statistically significant if the P value was less than 0.05. Software SPSS 13.0 was used in all statistical tests.
By using RT-PCR, the EGFR gene expression in gastric mucosal cells were detected in rats of normal group and model group as a weak signal but it was well-defined among other groups: model serum group, stomach serum group and gallbladder serum group. Compared with Model serum group, the serum in stomach serum group and gallbladder serum group appeared to up-regulate significantly the EGFR gene expression in gastric mucosal cells, P < 0.01, and obvious difference between stomach serum group and gallbladder serum group was found (P < 0.01). However, there was no difference between normal group and model group, P > 0.05 (Table 2; Figure 1).
According to the classical Traditional Chinese Medicine (TCM) theory, there is a particular relation between meridian acupoints and viscera and the functional activities of the organism can be regulated by acupuncture at the meridian acupoints. However, it is still unknown how the acupuncture regulates the functional activities of the organism, and what is essential for the relationship between meridian acupoints and viscera. The present study proved that the acupuncture at the stomach meridian acupoints could improve gastric mucosal protection mechanism and that it is a very effective cure for gastrointestinal diseases[15,16]. Acupuncture at acupoints of Sibai (ST2), Liangmen (ST21), and Zusanli (ST36), could produce certain ameliorative effect through the following mechanisms: augmentation of the gastric antrum, reinforcement of pressure on gastric pyloric sphincter, stimulation or inhibition of related gastrointestinal peptide secretion[17,18]. All of these have provided experimental evidence for the theory of “Particular relationship between gastric meridian and the stomach”. However, the functional mechanism of the repair of gastric mucosal lesion is not entirely clear, and the humoral factor of acupuncture and moxibustion effect is still unknown.
The mucosal lining of the gastrointestinal tract, especially the stomach, is easily exposed to a variety of exogenous injurious agents, including non-steroidal anti-inflammatory drugs and ethanol. Each of these agents either alone or in combination with others may induce mucosal injury. However, a number of in vivo and in vitro studies have demonstrated that the gastric mucosa of animals possesses the inherent capacity to repair after mild injury[19]. The cellular protective functions against damage maybe accomplished in several ways. There are evidences for participation of both the early phase of epithelial repair, known as restitution, marked by increased cell migration but no proliferation, and the delayed phase of cell renewal, marked by proliferation, differentiation and migration[20,21].
In general, EGFR is one of the recently described members of cell membrane proteins. It is made of 1186 amino acids. As a trans-membrane receptor of tyrosine protein kinase family, EGFR plays a very important role in regulating healing process of damaged gastric mucosa, and regulates cell metabolism, proliferation, differentiation, migration and other biological phenomena. Many studies indicated that there was an elevated EGFR expression during the healing course of damaged gastric mucosa. Therefore, EGFR is of a great importance to the gastric mucosal protection and injury healing[22,23]. The relationship between EGFR and its downstream signal transduction pathway and the healing of gastric mucosal injury is increasingly becoming a focus of researchers’ attention. This study assessed, by RT-PCR methods, the EGFR mRNA expression in gastric mucosal cells of the rat after incubation with 10% serum for 30 min. The data showed that EGFR mRNA expression in gastric mucosal cells was enhanced shortly after incubation with the serum derived from the rats. Meanwhile, it was proved that the serum derived from the rats treated with electroacupuncture had an obvious tendency to stimulate the EGFR mRNA expression in gastric mucosal cells. In addition, EGFR mRNA expression in stomach serum group was much higher than that in model serum group and gallbladder serum group. Therefore, we hypothesize that the serum derived from rats treated with electroacupuncture contains many kinds of active substances that stimulated the EGFR gene in the gastric mucosal cells. This study also indicated that the discrepancy in the expression of EGFR gene may be the underlying mechanism of different effect of electroacupuncture at acupoints of gastric meridian and that of gallbladder meridian. Thus, this could be a proof for the TCM theory “particular relation between SMFY and the stomach”. The active substance (s) in the serum derived from the rats treated with electroacupuncture at stomach meridian acupoints is (are) unknown, and therefore, more research using proteomic technology is needed.
S- Editor Liu Y L- Editor Karam SM E- Editor Bi L
| 1. | Wan Q. Auricular-plaster therapy plus acupuncture at zusanli for postoperative recovery of intestinal function. J Tradit Chin Med. 2000;20:134-135. [PubMed] |
| 2. | Butov MA, Alebastrov AP, Kuznetsov PS, Shirokova IV. [The age aspect of treatment of patient with ulcer of gastroduodenal zone]. Adv Gerontol. 2004;14:96-100. [PubMed] |
| 3. | Gönül B, Akbulut KG, Ozer C, Yetkin G, Celebi N. The role of transforming growth factor alpha formulation on aspirin-induced ulcer healing and oxidant stress in the gastric mucosa. Surg Today. 2004;34:1035-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Li H, Tang CZ, Li SH, Zhang Z, Chen SJ, Zhang JW. [Effects of thread embedding therapy on nucleotides and gastrointestinal hormones in the patient of chronic gastritis]. Zhongguo Zhen Jiu. 2005;25:301-303. [PubMed] |
| 5. | Chang CH, Huang JL, Ting CT, Chang CS, Chen GH. Atropine-induced HRV alteration is not amended by electroacupuncture on Zusanli. Am J Chin Med. 2005;33:307-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Tarnawski AS, Jones MK. The role of epidermal growth factor (EGF) and its receptor in mucosal protection, adaptation to injury, and ulcer healing: involvement of EGF-R signal transduction pathways. J Clin Gastroenterol. 1998;27 Suppl 1:S12-S20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 74] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 7. | Mendelsohn J. Targeting the epidermal growth factor receptor for cancer therapy. J Clin Oncol. 2002;20:1S-13S. [PubMed] |
| 8. | Ciardiello F, Tortora G. A novel approach in the treatment of cancer: targeting the epidermal growth factor receptor. Clin Cancer Res. 2001;7:2958-2970. [PubMed] |
| 9. | Konturek PC, Brzozowski T, Konturek SJ, Taut A, Sliwowski Z, Stachura J, Hahn EG. Activation of genes for growth factors and cyclooxygenases in rat gastric mucosa during recovery from stress damage. Eur J Pharmacol. 1998;342:55-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | He J, Yan J, Chang X, Liu J, Li J, Yi S, Lin Y. Neurons in the NTS of rat response to gastric distention stimulation and acupuncture at body surface points. Am J Chin Med. 2006;34:427-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Everett SM, White KL, Schorah CJ, Calvert RJ, Skinner C, Miller D, Axon AT. In vivo DNA damage in gastric epithelial cells. Mutat Res. 2000;468:73-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Xiao ZQ, Majumdar AP. Induction of transcriptional activity of AP-1 and NF-kappaB in the gastric mucosa during aging. Am J Physiol Gastrointest Liver Physiol. 2000;278:G855-G865. [PubMed] |
| 13. | Chen Y, Zhao C, Chen H, Qin H, Fang F. Effects of "moxibustion serum" on proliferation and phenotypes of tumor infiltrating lymphocytes. J Tradit Chin Med. 2003;23:225-229. [PubMed] |
| 14. | Luo MF, Li CH, Zhang JL, Guo Y, Chen SP, Liu JL, Li RW. [Acupuncture-serum decreases Ca2+ content in cultured rat myocardial cells]. Zhongguo Zhen Jiu. 2006;26:367-370. [PubMed] |
| 15. | Iwa M, Nakade Y, Pappas TN, Takahashi T. Electroacupuncture improves restraint stress-induced delay of gastric emptying via central glutaminergic pathways in conscious rats. Neurosci Lett. 2006;399:6-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Lee YH, Lee MS, Shin BC, Jeong JS, Jeong DM, Hwang IC, Kim JI. Effects of acupuncture on potential along meridians of healthy subjects and patients with gastric disease. Am J Chin Med. 2005;33:879-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Yan J, Yang RD, He JF, Yi SX, Chang XR, Lin YP. Effect of acupuncture at different meridian acupoints on changes of related factors for rabbit gastric mucosal injury. World J Gastroenterol. 2005;11:6472-6476. [PubMed] |
| 18. | Li XP, Yan J, Yi SX, Chang XR, Lin YP, Yang ZB, Huang A, Hu R. Effect of electroacupunture on gastric mucosal intestinal trefoil factor gene expression of stress-induced gastric mucosal injury in rats. World J Gastroenterol. 2006;12:1962-1965. [PubMed] |
| 19. | McNeil PL. Repairing a torn cell surface: make way, lysosomes to the rescue. J Cell Sci. 2002;115:873-879. [PubMed] |
| 20. | Sobue M, Joh T, Oshima T, Suzuki H, Seno K, Kasugai K, Nomura T, Ohara H, Yokoyama Y, Itoh M. Contribution of capsaicin-sensitive afferent nerves to rapid recovery from ethanol-induced gastric epithelial damage in rats. J Gastroenterol Hepatol. 2003;18:1188-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Rodríguez JA, Theoduloz C, Yáñez T, Becerra J, Schmeda-Hirschmann G. Gastroprotective and ulcer healing effect of ferruginol in mice and rats: assessment of its mechanism of action using in vitro models. Life Sci. 2006;78:2503-2509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 60] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Zhang WN, Zhang Y, Li JB. [Effect of Jianwei Yuyang Granules on expression of epidermal growth factor receptor in gastric mucosa of gastric ulcer patients]. Zhongxiyi Jiehe Xuebao. 2004;2:24-26. [PubMed] |
| 23. | Fujiwara Y, Higuchi K, Hamaguchi M, Takashima T, Watanabe T, Tominaga K, Oshitani N, Matsumoto T, Arakawa T. Increased expression of transforming growth factor-alpha and epidermal growth factor receptors in rat chronic reflux esophagitis. J Gastroenterol Hepatol. 2004;19:521-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |