INTRODUCTION
The concept that pericanalicular actin filaments may subserve a contractile function to facilitate bile flow was first proposed in 1974[1]. Contractile proteins are found to be present throughout the cytoplasm in hepatocytes and are particularly numerous in the pericanalicular region by electron microscopy[2,3], immunohistochemistry[4,5], and biochemical analysis[6]. Strong evidence for bile canalicular motility came from in vitro studies using isolated rat hepatocyte couplets (IRHC)[7,8], which indicated dynamic contractions of the bile canaliculi. The canalicular motility is impaired by actin inhibitors such as cytochalasin B and phalloidin[9,10]. Taurocholate, a choleretic bile acid, also increases the contraction rate, thus providing further support for a role of canalicular contractions in the bile secretory mechanism[11]. More importantly, canalicular contractions have been demonstrated in living rats[12], and this finding substantiates a number of in vitro data regarding the mechanisms of bile canalicular contractions.
Lithocholic acid, a hydrophobic secondary bile acid, is well known to cause intrahepatic cholestasis[13]. There have been extensive studies on the mechanisms of lithocholate-induced cholestasis in animals. Lithocholate diminishes both the bile acid-dependent and independent bile flow[14]. Regarding the mechanisms of the bile acid-independent bile flow, it has been explained by the inhibition of Na+, K+-ATPase[15,16], the inhibition of gap junction permeability[17], and the impairment of hepatic transporters such as the bile acid export pump (Bsep)[18-20]. In humans, elevated levels of lithocholic acid are found in patients with chronic cholestatic liver disease[21,22] . From an ultrastructural point of view, the lamellar transformation of the canalicular membranes reported by Miyai et al. has been considered the hallmark of lithocholic acid-induced cholestasis[23,24]. To elucidate the precise mechanism of cholestasis, we investigated the direct effects of taurolithocholate (TLC) on the canalicular motilities in IRHC using time-lapse cinephotomicrography and transmission electron micrography.
MATERIALS AND METHODS
Liver cell culture
Wistar strain female rats, weighing 170-230 g, were used. These rats were fed with a laboratory pellet rat diet and tap water ad libitum. All animals were given humane care in compliance with institutional guidelines. Primary isolated hepatocytes were prepared using the same method reported previously[8,9]. Briefly, liver perfusion and dissociation were based on the method of Seglen[25] as modified by Laishes and Williams[26]. After an initial washout perfusion was performed through the portal vein using Ca2+ and Mg2+ free Hanks balanced salt solution containing 0.5 mmol/L EGTA, the rat livers were then perfused with L-15 medium containing 0.05% type I collagenase (Sigma Chemical Company). One million isolated hepatocytes were inoculated into a 60 mm Corning culture dish (Corning Glass Works, Corning, NY), and then maintained with L-15 medium which contained 10% fetal bovine serum, 10 mmol/L HEPES, penicillin (100 U/L) and streptomycin (100 U/L). The viability of isolated hepatocytes by trypan blue exclusion test was approximately 95%. These cells were preincubated at 38°C for 4 h to facilitate cell attachment to the bottom of the dish and to allow time for recovery from the cell isolation procedure. The incompletely separated groups of cells, especially couplets and triplets, were selected for this experiment.
Time-lapse cinephotomicrography
A Nikon inverted microscope (Diaphot-TMD) with phase-contrast optics and a 16 mm movie camera (H16, RX-5 Bolex) operated by a Nikon cine auto-timer with a drive system (CFMA) were used. This equipment was housed in a temperature-controlled room maintained at 38°C. Time-lapse movies were taken at a speed of 1 frame/15 s for 12 h after the addition of TLC. Five time-lapse movies containing 3 representative bile canaliculi from 5 rats were taken for each of the experimental and control groups.
Experimental design and methods of analysis
In the experimental groups, sodium taurolithocholate (grade A, purity > 99%, Calbiochem, San Diego, CA) was added to the culture media at concentrations of 10 and 50 μmol/L, respectively. The TLC solution was prepared from the stock solution containing 20 mmol/L TLC dissolved in propylene glycol. In the controls, the same solution without TLC was added to the culture media. Three representative bile canaliculi in each movie were used for analysis. A total of 2880 frames, i.e., 12 h of real time were examined in each canaliculus using an analytic movie projector (Photo-Optical Data Analyzed 2240A, MK-V, L-W International, Woodland Hills, CA). Bile canalicular contraction was defined as a visible decrease in canalicular diameter. The minimum size of the canalicular lumen was taken as the end point of the contraction. Analysis of the movies was performed using the frame numbers of the movie films at the point of beginning and end of the contractions. The time intervals between adjacent contractions were calculated as the difference in the frame counts between consecutive contractions. These frame counts were then converted into real time.
Statistical analysis
The frame numbers obtained from all the movies were stored and analyzed by a personal computer. The number of bile canalicular contractions was determined for 3 canaliculi from each movie of 5 rats and evaluated by analysis of variance test for each dose of TLC used. The intervals between consecutive canalicular contractions were calculated and compiled to make a histogram. The histograms of 10 and 50 μmol/L TLC and controls were assessed statistically, presuming that these data have a Poisson distribution as reported previously[8,11].
Electron microscopy
Representative samples of IRHC after the addition of 10 and 50 μmol/L TLC and the controls were examined by transmission electron microscopy. The cells cultured on plastic cover slips were fixed in universal fixative[27] at 1, 3 and 6 h after the addition of either TLC or propylene glycol. For postfixation, 1% osmium tetroxide in 0.1 mol/L cacodylate buffer (pH 7.4) for 30 min was used. The cells were dehydrated in a graded series of alcohol and embedded in Epon 812. Ultrathin sections were cut using LKB ultramicrotome and stained with Sato’s lead solution[28]. The specimens were examined using a Philips EM 400T electron microscope (Philips Electronic Instruments Inc., Mahwah, N.J.).
RESULTS
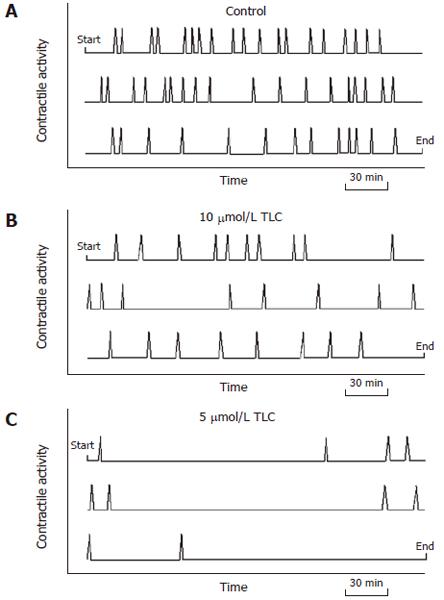
Remarkable motilities of isolated hepatocyte couplets and triplets were observed in the time-lapse movies. In the controls, bile canaliculi revealed spontaneous and forceful contractions (Figure 1). The canalicular contractions were most striking for cell motility, while Brownian-like movement and cell ruffling were also seen. Active movements of small particles, which may represent cytoplasmic vesicles and vacuoles, were noted in the pericanalicular regions of the cytoplasm. TLC-treated hepatocytes manifested a marked reaction to this bile acid as compared with the controls. Immediately after the addition of TLC, the bile canaliculi were deformed, and canalicular bile was incorporated into the vacuoles in hepatocyte cytoplasm. The canaliculi were gradually dilated, and canalicular contractions were markedly inhibited by TLC (Figure 2). This phenomenon was most prominent with a high dose (50 μmol/L) of TLC. Some bile canaliculi showed only a few contractions approximately 6 h after the addition of TLC. The pericanalicular movements of the small particles observed by phase-contrast microscopy became extremely slow with the progression of time. A number of cytoplasmic vesicles and vacuoles were found in the pericanalicular region. The graphic displays of representative canalicular contractions in the controls and the TLC-treated groups are shown in Figure 3. Whereas canalicular contractions occurred at regular intervals in the control groups, the contraction activity was visibly diminished, as the TLC dosage increased.
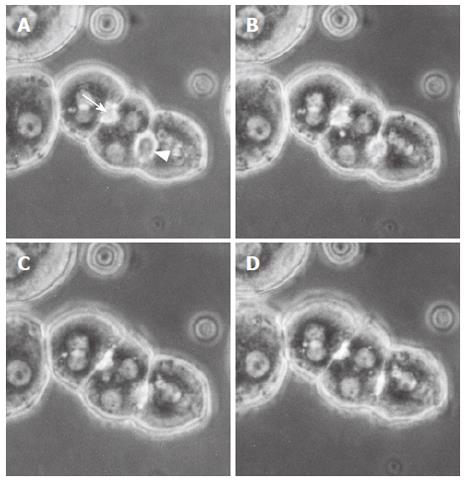
Figure 1 Representative phase contrast micrographs of an isolated hepatocyte triplet in the controls.
Figures A, B, C and D show a sequence of the bile canalicular contractions. A bile canaliculus indicated by an arrowhead started to contract (A) and completed contraction (D). Another canaliculus indicated by an arrow started its contraction (B) and completed contraction (C). These canalicular contractions were forceful and repetitive.
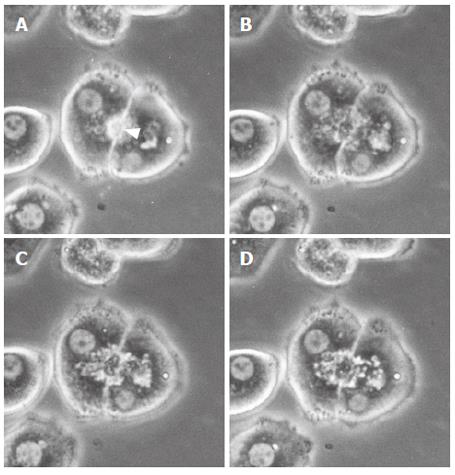
Figure 2 Representative phase contrast micrographs of an isolated hepatocyte couplet in the taurolithocholate (TLC)-treated groups (50 μmol/L).
Prior to addition (A) and after the addition of TLC (B, C, D). A canaliculus (arrowhead) between two hepatocytes (A) was deformed, and canalicular bile was incorporated into the vacuoles in the hepatocyte cytoplasm immediately after the addition of TLC (B). Thereafter, the bile canaliculi were gradually dilated, and the canalicular contractions were markedly impaired (C, D). In the TLC-treated groups, the movements of small particles became extremely slow, and cytoplasmic vesicles and vacuoles were noted in the pericanalicular region.
Figure 3 Graphic display of the representative contraction activity of bile canaliculi.
Diagrams represent the 12 h period of observation, beginning at the upper left corner and ending at the lower right corner. The canalicular contractions (spikes) occurred at regular intervals in the control groups (A). The contraction activity visibly diminished as the TLC dosage increased (B, C).
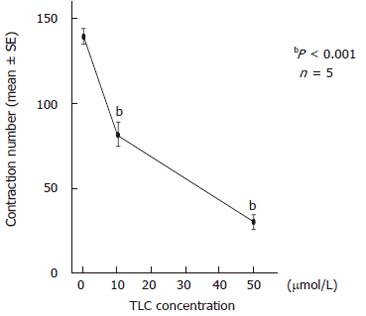
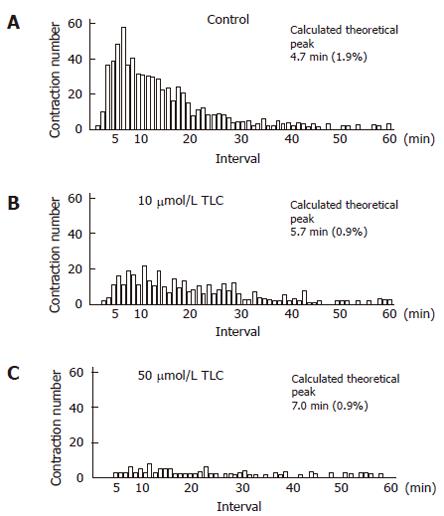
To examine the detailed functions of the canalicular contractions, we analyzed the number of contractions and the contraction interval for 12 h. The total contraction number of 3 bile canaliculi in the TLC-treated groups significantly decreased to 82.4 ± 6.1 (mean ± SE) at 10 μmol/L and 30.2 ± 3.1 at 50 μmol/L, as compared with that in the control groups (139.4 ± 4.5) (Figure 4). The histogram of the canalicular contraction interval revealed the Poisson distribution. In the TLC-treated groups, there was a tendency for the peak height to decrease and shift to the right. In other words, the time intervals between adjacent contractions were prolonged, as the TLC dosage increased. The calculated theoretical peak values, which reflect the maximum likelihood of the next contraction, were 4.7 min in the control groups, 5.7 min at 10 μmol/L and 7.0 min at 50 μmol/L TLC (Figure 5).
Figure 4 Effects of taurolithocholate (TLC) on the contraction number of bile canaliculi.
The total contraction number of 3 bile canaliculi for 12 h was 139.4 ± 4.5 (mean ± SE) in the controls, and it significantly decreased to 82.4 ± 6.1 at 10 μmol/L TLC and 30.2 ± 3.1 at 50 μmol/L. The contraction number markedly decreased as the TLC dosage increased.
Figure 5 Effects of taurolithocholate (TLC) on the contraction interval of bile canaliculi.
The histogram of the canalicular contraction interval revealed Poisson distribution. In the TLC-treated groups, there was a tendency for the peak height to decrease and shift to the right. The calculated theoretical peak values for each dose were 4.7 min in the control groups (A), 5.7 min at 10 μmol/L TLC (B) and 7.0 min at 50 μmol/L (C). Normalized errors are indicated in parentheses.
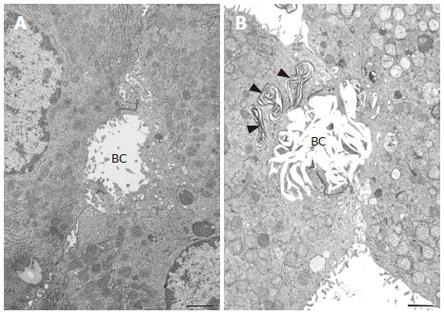
Transmission electron microscopy revealed that IRHC prior to the addition of TLC showed either normal or slightly dilated bile canaliculi. The canaliculi between two hepatocytes were filled with microvilli and sealed by tight junctions. A rich network of microfilaments, i.e., actin filaments were recognized around the canaliculi. It was noted that TLC caused characteristic lamellar transformation of the bile canalicular membranes (Figure 6). This lamellar structure became more prominent and widely spread with the progression of time after the addition of TLC. The bile canaliculi were deformed and tended to become dilated with the loss of microvilli. Electron dense membranous structures of the biliary materials were found to be incorporated into the vacuoles in the hepatocyte cytoplasm, especially in the pericanalicular region.
Figure 6 Transmission electron micrographs of isolated hepatocytes.
A bar indicates 1 μm. A: Normal hepatocytes prior to the addition of taurolithocholate (TLC). A bile canaliculus (BC) was slightly dilated and filled with microvilli. B: 12 h after the addition of 50 μmol/L TLC. Note the characteristic lamellar transformation of the bile canalicular membranes. A bile canaliculus (BC) was deformed and dilated with a loss of microvilli. Electron dense membranous structures of biliary materials (arrowheads) were found in the vacuoles in the pericanalicular region.
DISCUSSION
The present study using time-lapse movies permits the continuous recording of the dynamic cell motilities in IRHC. The bile canaliculi between adjacent isolated hepatocytes manifested a marked reaction to TLC. The canaliculi were immediately deformed after the addition of TLC, and thereafter they became dilated with the marked impairment of the canalicular contractions. The contraction number of the canaliculi significantly decreased to 82.4 ± 6.1 at 10 μmol/L and 30.2 ± 3.1 at 50 μmol/L, as the TLC dosage increased. Since Phillips and his co-workers demonstrated dynamic contractions of the bile canaliculi[7,8], a number of studies using IRHC have been performed to elucidate the mechanisms of the bile canalicular contraction. The results indicate the active contraction process of the bile canaliculi mediated by the pericanalicular contractile proteins. It is notable that the contractions are Ca2+, calmodulins and ATP-dependent [4,9,10,29]. Hence, the mechanism of canalicular contraction has features in common with the actin-myosin based cytoplasmic motility behavior found in other non-muscle cells. The canalicular contractions are spontaneous but highly ordered. In triplets, when one bile canaliculus contracts, a neighboring canaliculus is likely to contract after an interval of 1.25 min, thus indicating a coordination in the contractile activity of the BC[30]. In the present study, the BC in the control couplets and triplets showed spontaneous and forceful contractions. The total contraction number of 3 bile canaliculi was 139.4 ± 4.5 in the control groups, which is almost equal to the findings of previous reports[9-11].
The permeability of cell membranes is greatly influenced by cholesterol, including specific changes in the membrane cholesterol affected by both the functional properties of the lipid bilayer and the function of membrane-bound proteins[31-33]. In physiological conditions, cholesterol has been viewed as a modulator of membrane fluidity and permeability[34,35]. The alterations in membrane permeability or fluidity in the liver have been considered to be one of the mechanisms of intrahepatic cholestasis[36]. In this regard, lithocholate-induced cholestasis is of special interest, since the canalicular membrane itself is the site of cholesterol deposition[16,23]. A biochemical analysis of canalicular enriched isolated plasma membrane preparations revealed an eight-fold increase in cholesterol in TLC-induced intrahepatic cholestasis[24]. It is therefore anticipated that pathological alterations in cholesterol of the canalicular membranes may affect the membrane structure and function, especially bile canalicular contractions.
In this study, a canaliculus between adjacent isolated hepatocytes manifested a marked reaction to TLC. Immediately after the addition of TLC, the bile canaliculi were deformed and gradually dilated, leading to a remarkable reduction in the canalicular contraction number. The prompt responses of the bile canaliculi to TLC suggest that TLC may directly act on the canalicular membranes, resulting in the alteration and dysfunction of the canaliculi. TLC is mainly taken up by the hepatocytes through the Na+ taurocholate cotransporting polypeptide (Ntcp)[37], and the canalicular secretion of TLC is mainly mediated by Bsep[38]. It is therefore speculated that TLC taken up by IRHC may directly act on the canalicular membranes, resulting in the formation of the lamellar structure as recognized by electron microscopy. In normal IRHC, a rich network of microfilaments is found in the pericanalicular region, and the circumferential band of microfilaments surrounding the bile canaliculi is considered to fulfill a contractile function[4]. Since the ultrastructural alterations of the pericanalicular microfilaments were not evident after the TLC treatment in this study, it is considered that the impairment of the canalicular contraction in IRHC may thus be due to the abnormalities of the canalicular membranes themselves, without affecting the cytoskeletal organization around the bile canaliculi.
It is likely that the canalicular motility events are related to bile secretion. We analyzed the contraction interval of the bile canaliculi to elucidate the effect of TLC on the canalicular secretory function by the same method as previously reported[11]. The histogram of the canalicular contraction interval indicates that there was a tendency for the peak height to decrease and shift to the right, as the TLC dosage increased. The calculated theoretical peak values, which reflect the maximum likelihood of the next contraction, were 4.7 min in the control groups, 5.7 min at 10 μmol/L and 7.0 min at 50 μmol/L TLC, indicating that the time intervals between adjacent contractions are prolonged by TLC. The bile canaliculi are the smallest biliary passages across which bile secretion occurs. Taurocholic acid causes a linear increase in bile secretion in IRHC, suggesting that canalicular contractions may be a function of canalicular bile secretion[11]. The present study demonstrates that TLC impairs not only the bile canalicular contractions, but also canalicular bile secretion. The reduction in the contractions after a lag period probably indicates that secretion is impaired, and hence the need for contractions is reduced. Since colchicines, an inhibitor of microtubules, also inhibits both the canalicular contractions and bile secretion in IRHC[39], these results therefore support the view that canalicular motility is closely associated with canalicular bile secretion.
Recent research on bile secretion has focused on the characterization of hepatobiliary transporters such as Ntcp and Bsep, indicating that TLC impairs the expression of Bsep[18-20]. Bsep is a major bile acid transporter in the liver, and mutations in Bsep result in progressive intrahepatic cholestasis. Crocenzi et al. reported that TLC impairs bile salt secretion at the canalicular level and induces the internalization of Bsep into a cytosolic vesicular compartment without affecting the cytoskeletal organization in IRHC treated with TLC[20]. Bsep expression has also been reported to be regulated by ligands of the nuclear receptor farnesoid X receptor (FXR), and TLC decreases the expression of Bsep through FRX[19]. Taking all of these findings into consideration, the reduction of the canalicular secretion recognized in the present study may thus be associated with the decreased expression of Bsep in addition to the alterations in membrane permeability. The relationship of Bsep expression to the canalicular membrane permeability is still unknown, so the answer to this question must await further studies. In conclusion, TLC directly alters the bile canalicular membranes, thereby impairing both the canalicular motility and canalicular bile secretion.