Published online Sep 7, 2006. doi: 10.3748/wjg.v12.i33.5293
Revised: April 28, 2006
Accepted: May 22, 2006
Published online: September 7, 2006
AIM: To assess the efficacy of triple therapy (peginte-rferon or high dose standard interferon, plus ribavirin and amantadine) in nonresponders to prior combination therapy.
METHODS: A total of 196 patients were enrolled in a multicenter, open, randomized study. Patients were given 180 μg/wk of peginterferon-alpha-2a (40 kDa) plus ribavirin (800-1000 mg/d) and amantadine (200 mg/d) for 48 wk (group A) or interferon-alpha-2a (6 MU/d for 4 wk, 3 MU/d for 20 wk, and 3 MU tiw for 24 wk) plus ribavirin (800-1000 mg/d) and amantadine (200 mg/d) for 48 wk (group B).
RESULTS: Overall sustained virologic response (SVR) was 26.6% (32.1% and 19.5% in group A and B, P = 0.057). Baseline ALT >120 UI/L (OR 2.4; 95% CI:1.11 to 5.20; P = 0.026) and HCV RNA negativity after 12 wk (OR 8.7; 95% CI: 3.87 to 19.74; P < 0.0001) were independently associated with SVR. Therapy discontinuation occurred less frequently in patients treated with peginterferon than standard interferon (P = 0.036).
CONCLUSION: More than 25% of nonresponders to combination therapy can eradicate HCV infection when retreated with triple therapy, especially if they have a high baseline ALT and are treated with pegylated interferon.
- Citation: Fargion S, Borzio M, Maraschi A, Cargnel A, Lombardo TPOTGE. Triple antiviral therapy in HCV positive patients who failed prior combination therapy. World J Gastroenterol 2006; 12(33): 5293-5300
- URL: https://www.wjgnet.com/1007-9327/full/v12/i33/5293.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i33.5293
While advances in the treatment of HCV chronic hepatitis have markedly improved outcomes for treatment-naïve patients, a large number of patients still fail to eradicate HCV infection[1-4], and improving the re-treatment success rates of these nonresponsive patients remains a key challenge in hepatitis care[5-7]. It has been reported that 12%-20% of patients who did not respond to standard interferon (IFN) monotherapy can achieve a sustained response when retreated with high doses of interferon plus ribavirin (RBV)[8-12]. However, the most effective treatment strategies for patients who fail to respond to combination therapy have yet to be established, and results for re-treatment with pegylated interferon and ribavirin in these patients are generally more variable and less positive[13-17]. The results can also be difficult to interpret since most of the available data derive from studies that included both relapsers and nonresponders to different therapeutic schedules. However, considering the high number of patients who do not respond to combination therapy, the need for new antiviral re-treatment regimens becomes evident.
It has recently been suggested that the addition of amantadine (AMA) to interferon and ribavirin (triple therapy) may improve the therapeutic efficacy of combination therapy, possibly by potentiating the antiviral activity through interleukin production[18]. Amantadine has an intrinsic antiviral activity against influenza A virus by blocking its cellular internalization and possibly interferes with the replication of other viruses including HCV[19-21]. Results on the use of triple therapy in patients with HCV chronic hepatitis are conflicting. A recent meta-analysis[22] reported that triple therapy was of no advantage compared to standard combination therapy in treatment-naive patients or in relapsed patients[23,24], whereas it markedly improved the sustained virologic response in nonresponders to interferon monotherapy[25-27]. The efficacy of triple therapy in nonresponders to combination therapy has primarily been investigated in only a small series of patients, making it difficult to draw defined conclusions from these data on the use of triple therapy in such patients[28-32]. A recent study in a larger patient population of nonresponders (n = 200) found a higher sustained virological response in patients receiving triple therapy (24%) vs combined therapy (16%), although the difference was not statistically significant[33].
The aims of this multicenter, randomized study carried out in a well-selected series of patients nonresponsive to combination therapy were (1) to define the rate of sustained virological response in patients previously nonresponsive to combination therapy, when retreated with triple therapy (consisting of recombinant interferon or peginterferon (PegIFN) plus ribavirin and amantadine); (2) to assess whether PegIFN is superior to a high daily dose of IFN when used in a triple therapy regimen; and (3) to compare the tolerability, side effects and adherence to therapy in patients treated with the two different schedules.
From February 2001 to June 2002, 196 patients aged between 18 and 65 years were included in this multicenter cooperative study. All patients had to be nonresponders to interferon plus ribavirin given for at least 24 wk. Patients who had previously been treated with interferon alone or who had received combination therapy for a shorter period were excluded from the study. Patients were recruited by 21 Liver Centers operating in 21 General Hospitals sited in Lombardy (North of Italy) and belonging to the Gruppo Epatologico Lombardo (GEL). A noncompetitive recruitment was employed and each center had to enroll at least 5 patients. The failure to respond to previous combination therapy was defined as absence of normalization of transaminases and/or detectable serum HCV RNA level after 24 wk of therapy. A washout period of at least 6 mo from the end of therapy to inclusion in this study was required. For inclusion, patients had to fulfill the following criteria: (1) persistent transaminase elevation throughout the previous 6 mo; (2) a liver biopsy taken within the past 18 mo showing histological findings compatible with chronic hepatitis or cirrhosis; (3) serum HCV RNA positive by quantitative polymerase chain reaction (PCR) test (second-generation Amplicor HCV, Roche Diagnostic System, Basel, Switzerland). Patients were excluded from the study if one of the following criteria were present: (1) decompensated cirrhosis; (2) co-infection with human immunodeficiency virus; (3) positivity for HBsAg; (4) autoimmune hepatitis; (5) alcohol abuse (daily alcohol intake > 60 g in males and > 40 g in females); (6) haemoglobin concentration less than 120 g/L in women and 130 g/L in men; (7) white cell count less than 3 × 109/L, platelet count below 100 × 109/L; (8) moderate/severe depression or other psychiatric diseases; (9) seizure disorders; (10) cardiovascular, respiratory or renal clinically manifested diseases; (11) hemoglobinopathies; (12) poorly controlled diabetes mellitus; (13) immunologically mediated disease; (14) ultrasonographic evidence of focal liver lesions.
This was a multicenter, open-label, randomized, parallel-group study. Randomization was centralized and patients were stratified by HCV genotype (genotype 1 vs other genotypes).
Patients were randomized to receive peginterferon-α-2a (Pegasys; Roche, Basel, Switzerland) at the dose of 180 mcg once a wk for 48 wk (Treatment A) or interferon alfa-2a (Roferon; Roche) at the dose of 6 MU/d for 4 wk followed by 3 MU/d for the next 20 wk and 3 MU tiw until the end of the study (wk 48) (Treatment B). Both groups received ribavirin (Copegus, Roche, Basel, Switzerland) at the dose of 800 mg (for patients weighing less than 75 kg) or 1000 mg (for patients weighing 75 kg or more) and amantadine (Mantadan; Boehringer Ingelheim, Florence, Italy) at a total daily dose of 200 mg. The treatment period lasted 48 wk, with a 24-wk follow-up period. Sustained virological response was defined as persistent negative HCV RNA at 6 mo after completion of therapy. Patients were considered nonresponders and therapy was stopped if HCV-RNA was still positive at 24 wk of therapy.
Basal laboratory assessment consisted of liver function tests, hematologic tests, renal function tests, autoantibodies (ANA, AMA, ASMA, antiLKM), alphafetoprotein, serum iron, transferrin and ferritin, HBsAg, anti-HBc. Quantitative HCV RNA was measured by standardized PCR assay with a lower limit of detection of less than 1 × 106 copies/L (second-generation Amplicor HCV Monitor, Roche), qualitative HCV RNA was measured by a standardized PCR assay with a lower limit of detection of 0.1 × 103 copies/L (second-generation Amplicor HCV, Roche Diagnostic System). Determination of infecting HCV genotype was performed in serum samples prior to therapy using Innolipa, Genetics Technique (Innogenetics, Belgium/Bayer Diagnostics, USA).
Patients were clinically evaluated at regular intervals: 15, 30, 45 and 60 d after the first dose of treatment and every month thereafter until the end of the study. Biochemical tests were performed at 15 and 30 d and at monthly intervals until the end of treatment and every 3 mo during follow-up. During treatment, HCV-RNA was assayed after 12, 24, 36, and 48 wk of therapy and after 12 and 24 wk from the end of the treatment. At 12 and 72 wk, the serum HCV RNA level was assayed by qualitative analysis.
Liver biopsies were formalin fixed, embedded in paraffin and stained by hematoxylin-eosin, reticulin and Masson’s trichrome method. Histologic diagnosis was made by one independent pathologist and classified according to the internationally accepted criteria as chronic hepatitis (mild, moderate or severe) or cirrhosis. Inflammatory activity (grading) and fibrosis (staging) were semi-quantified using the Ishak scoring system[34]. Local institutional committees of participating hospitals approved the study protocol and all amendments. All patients provided written informed consent. All the study procedures were in accordance with the principles of the Helsinki Declaration.
Clearance of HCV RNA at the end of the 6-mo follow up period was the primary end-point. Safety, adherence to therapy and therapy interruption were considered as secondary end-points.
Safety was assessed by physical examinations, laboratory tests and spontaneous reports of clinical adverse events. According to the study protocol, Peginterferon-α-2a dose modification to 135, 90 or 45 mg/wk and ribavirin dose reductions were allowed in patients with clinically significant adverse events or laboratory abnormalities, including hematological toxicity as manifested by the hemoglobin level of less than 100 g/L, white cell count of less than 2.5 × 109/L, granulocyte count of less than 1 × 109/L, and platelet count of less than 70 × 109/L. Therapy was discontinued in patients with neutrophils below 0.7 × 109/L, Hb below 85 g/L, and platelets below 50 × 109/L. Adherence to treatment was considered when drug consumption (either pegylated/standard IFN or ribavirin or both) was higher than 70% of the scheduled dose for a time period longer than 70% of the scheduled time.
It was calculated that at least 100 patients per group were needed to detect a difference of 20% in the proportion of SVR between treatment A and treatment B with an alpha error of 0.05 and study power (beta error) of 0.8. Summary statistics (no. of cases, mean, median, standard deviation, minimum and maximum) were calculated for continuous variables, and the number and percentage of patients in each category were provided for categorical data. The treatment comparison of interest was the recombinant interferon-α-2a plus ribavirin plus amantadine against peginterferon-α-2a plus ribavirin plus amantadine. Differences between the treatments were assessed by the Cochran-Mantel-Haenszel test stratified according to HCV genotype (HCV genotype 1 vs other genotypes). The relationship between patients’ baseline characteristics and SVR was examined by logistic regression analyses. Univariate logistic regressions were used to confirm the importance of previously identified prognostic factors. To assess the independence of these factors, a backward elimination procedure was then undertaken using the factors that were significant in the univariate analyses. A log-linear model was used to investigate the interactions between HCV-RNA eradication after 12 wk of therapy and the variables predictive of sustained virologic response. All P values reported are two-sided.
All patients who received at least one dose of study medication were included in all efficacy analyses, and if they had undergone at least one safety assessment after baseline, they were included in the safety analysis. Patients with missing data in the primary or secondary efficacy endpoints were considered as nonresponders.
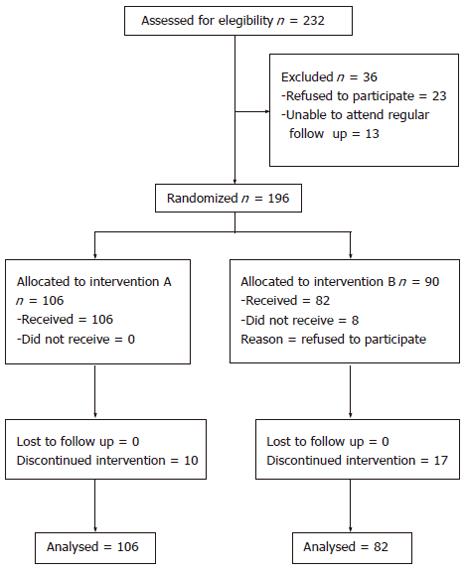
Two hundred thirty two consecutive nonresponder patients to combination therapy were considered eligible for this study. Thirty six patients were not randomized because of refusal of therapy (23 cases) or inability to attend a regular follow up (13 cases). One hundred six patients were randomized to receive peginterferon-α-2a plus ribavirin and amantadine and 90 to receive interferon α-2a plus ribavirin and amantadine. Eight patients randomized to treatment B refused to take the study drugs and were not further evaluated (Figure 1). The characteristics of patients at baseline are shown in Table 1. None of the variables considered was statistically significantly different between the two groups.
| Characteristic | All(n = 188) | PegIFN +RBV + AMA(n = 106) | IFN+RBV+AMA(n = 82) |
| Male/Female | 146/42 | 79/27 | 67/15 |
| Age | |||
| ≤ 40 yr | 42 (22) | 23 (22) | 19 (23) |
| > 40 yr | 146 (78) | 83 (78) | 63 (77) |
| BMI | |||
| ≤ 25 kg/m2 | 107 (64) | 60 (65) | 47 (63) |
| > 25 kg/m2 | 61 (36) | 33 (35) | 28 (37) |
| ALT | |||
| ≤ 120 UI/L | 107 (62) | 64 (65) | 43 (58) |
| > 120 UI/L | 66 (38) | 35 (35) | 31 (42) |
| HCV genotype | |||
| 1 | 147 (78) | 82 (77) | 65 (79) |
| 2 | 21 (11) | 12 (11) | 9 (11) |
| 3 | 8 (4) | 7 (7) | 1 (1) |
| 4 | 12 (6) | 5 (5) | 7 (9) |
| HCV RNA | |||
| ≤ 2 x 106 copies/mL | 74 (73) | 41 (76) | 33 (69) |
| > 2 x 106 copies/mL | 28 (27) | 13 (24) | 15 (31) |
| Stage | |||
| ≤ 3 | 127 (69) | 71 (70) | 56 (68) |
| > 3 | 56 (31) | 30 (30) | 26 (32) |
| Grade | |||
| ≤ 10 | 167 (92) | 94 (91) | 73 (92) |
| > 10 | 15 (8) | 9 (9) | 6 (8) |
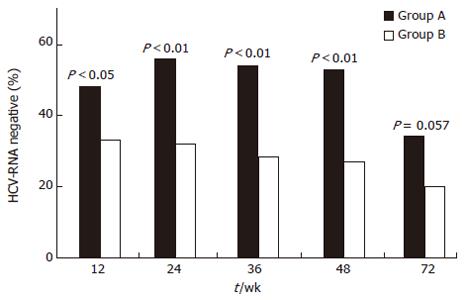
A SVR of 26.6% was obtained in the overall series of patients. However, when the two treatments were compared, a better but not significant SVR was achieved in patients receiving PegIFN than in those receiving high doses (daily doses for 6 mo) of standard interferon (32.1% vs 19.5%, P = 0.057). The better response in patients treated with PegIFN was present at all on-treatment time intervals (Figure 2). The rate of sustained response in the 30 patients with cirrhosis was 30% with no difference between patients treated with PegIFN or standard IFN.
Univariate analysis performed on non-missing data, considering the overall series of patients, showed that genotype non 1, higher grade score and baseline ALT were significantly associated with sustained virologic response (Table 2). Patients younger than 40 years had a significantly better response when treated with PegIFN than standard IFN (48% vs 11%, P = 0.017). Also patients with fibrosis less than 3 and those with viremia higher than 2 million copies had a significantly better response when treated with PegIFN than standard IFN (respectively 38% vs 18%, P = 0.018 and 46% vs 7%, P = 0.029) (Table 3).
| Characteristics | Sustained virologic response | P1 | |
| Genotype 1 | 32/147 | 22% | 0.0085 |
| Non 1 | 18/41 | 44% | |
| Age ≤ 40 yr | 13/42 | 31% | NS |
| > 40 yr | 37/146 | 25% | |
| BMI ≤ 25 kg/m2 | 32/107 | 30% | NS |
| > 25 kg/m2 | 12/61 | 20% | |
| Stage ≤ 3 | 37/127 | 29% | NS |
| > 3 | 12/56 | 21% | |
| Grade ≤ 10 | 42/167 | 25% | 0.03 |
| > 10 | 8/15 | 53% | |
| ALT ≤ 120 UI/L | 22/107 | 21% | 0.03 |
| > 120 UI/L | 24/66 | 36% | |
| HCV-RNA ≤ 2 × 106 copies/mL | 23/74 | 31% | NS |
| > 2 × 106 copies/mL | 7/28 | 25% | |
| Previous Total IFN ≤ 500 MU | 20/91 | 22% | NS |
| > 500 MU | 25/82 | 30% | |
| Characteristic | PegIFN+RBV+AMA | IFN+RBV+AMA | P | ||
| Genotype 1 | 21/82 | 26% | 11/65 | 17% | NS |
| Non 1 | 13/24 | 54% | 5/17 | 29% | NS |
| Age ≤ 40 yr | 11/23 | 48% | 2/19 | 11% | 0.017 |
| > 40 yr | 23/83 | 28% | 14/63 | 22% | NS |
| BMI ≤ 25 kg/m2 | 22/60 | 37% | 10/47 | 21% | NS |
| > 25 kg/m2 | 8/33 | 24% | 4/28 | 14% | NS |
| Stage ≤ 3 | 27/71 | 38% | 10/56 | 18% | 0.018 |
| > 3 | 6/30 | 20% | 6/26 | 23% | NS |
| Grade ≤ 10 | 28/94 | 30% | 14/73 | 19% | NS |
| > 10 | 6/9 | 67% | 2/6 | 33% | NS |
| ALT ≤ 120 UI/L | 17/64 | 27% | 5/43 | 12% | NS |
| > 120 UI/L | 15/35 | 43% | 9/31 | 29% | NS |
| HCV-RNA ≤ 2 × 106 copies/mL | 15/41 | 37% | 8/33 | 24% | NS |
| > 2 × 106 copies/mL | 6/13 | 46% | 1/15 | 7% | 0.029 |
The chance of achieving a sustained response was significantly higher in patients who were HCV RNA negative as compared to those who were HCV RNA positive at 12 wk of therapy (39/78, 50% vs 11/110 10%, P < 0.0001).
To examine the influence of potentially important prognostic factors on SVR, factors known to affect response (HCV genotype, age, BMI, stage and grade, ALT value, previous IFN total dose taken, biochemical and virologic response after 12 wk of treatment) were first examined individually by univariate logistic regression analysis for each factor for all treatment groups combined. ALT (< 120 UI/L vs > 120 UI/L), the biochemical and the virologic response at wk 12 were associated with SVR (P < 0.10). These predictive factors were entered in the final backward regression analysis. Two factors independently and significantly increased the odds of achieving a sustained virologic response: a baseline ALT value greater than 120 UI/L (odds-ratio 2.40; 95% CI: 1.11 to 5.20; P = 0.026), and the virologic response after 12 weeks of therapy (odds-ratio 8.75; 95% CI: 3.87 to 19.74; P < 0.0001). Logistic regression analysis was used to characterize further the relation between SVR, patients’ characteristics at baseline, biochemical and virologic response after 12 wk of treatment, and the study treatments. In the final backward regression analysis were entered the treatment given, the ALT value at baseline, the biochemical and the virologic response after 12 wk of treatment. The model confirmed that the baseline ALT value greater than 120 UI/L and the virologic response after 12 wk of treatment were prognostic factors that significantly increased the odds of achieving a sustained virologic response. Odds-ratios and their 95% confidence intervals and the associated p-values were equal to those obtained in the logistic regression model reported above.
Twenty-seven patients discontinued the study treatments: 10 in group A and 17 in group B (9.4% vs 20.7%, P = 0.036). The reasons for discontinuation are reported in Table 4. Dosage reduction, reported in 30 patients (17 treated with PegIFN and 13 with standard IFN), was related to hematological alterations in 12 of the patients who received pegylated interferon and in 7 of those treated with standard interferon. Dermatological manifestations (mainly reported in patients treated with pegylated interferon) and cephalea were the causes of dosage reduction in the remaining patients. Adherence lower than 70% in the first 24 wk of therapy was observed in 8/106 (7.5%) patients in treatment A and in 8/82 (10%) in treatment B. No significant difference in the rate of response was observed between patients who did or did not reduce therapy.
| PegIFN +RBV+ AMA(n = 106) | IFN+RBV+AMA(n = 82) | |
| Treatment discontinuation for any | 10 (9.4%) | 17 (20.7%) |
| Reason1 | ||
| Study drug noncompliance | 2 | 11 |
| Demyelinizing neuropathy | 1 | |
| Depression | 1 | 1 |
| Erythema | 1 | |
| Pulmonary fibrosis | 1 | |
| Hyperthyroidism | 1 | |
| Hyperthyroidism & Insomnia | 1 | |
| Increased ALT | 1 | |
| Leukopenia | 1 | 3 |
| Dyspnoea, Cough, Pruritus | 1 | |
| Unknown reason | 1 |
This is the first randomized study to compare the efficacy of two triple therapy regimens-recombinant interferon α-2a either standard or pegylated, in combination with ribavirin and amantadine-in a well-selected series of patients nonresponsive to previous treatment with interferon and ribavirin. Our data demonstrate that after 12 mo of treatment, a sustained virologic response is obtained in a surprisingly high number of these difficult-to-treat patients. Better results in terms of sustained response (32% vs 19% of SVR) were observed in patients treated by pegylated interferon than in those treated by a high daily dose of standard interferon. Baseline elevated ALT level and undetectable serum HCV RNA at wk 12 of therapy were the independent predictors of sustained response, irrespective of the interferon used (pegylated interferon or high dose standard interferon). As a whole, triple therapy was well tolerated and safe, with a higher frequency of therapy discontinuation in patients treated by standard interferon than in those treated by pegylated interferon.
The overall rate of SVR observed in the present study of nonresponders to combination therapy (standard interferon plus ribavirin), most of whom had been already submitted to several treatments, is higher than that generally observed in previously reported studies. The results are even more impressive when considering patients treated by pegylated interferon. In particular, patients with high viral load seemed to respond better when treated with pegylated interferon, even if the small number of cases in which basal viral load was available does not allow definite conclusions to be drawn. Only a few studies have been carried out to assess the efficacy of retreatment in patients nonresponsive to combination therapy and most of them included small series of patients and did not disaggregate relapsers from true nonresponders[24,25,27]. Schiffmann et al[14] reported an overall SVR rate of 18% after retreatment for 48 wk with PegIFN plus RBV, and even lower rates were observed in patients with unfavourable predictors such as genotype 1 and/or high viral load. Teuber et al[28] reported a 22% SVR in previous nonresponders either to interferon or combination therapy when retreated with triple therapy including amantadine, however, the SVR rate dropped down to 12% in those patients who had previously been given two or more courses of antiviral treatment. Typically, SVR rates in nonresponders to combination therapy retreated with triple therapy have averaged approximately 12%[29-32], although in a recent placebo-controlled study the SVR with amantadine triple therapy was 24%[33], while in a small group of genotype 1, nonresponsive patients without cirrhosis, a SVR of 42% was reported[35].
We cannot exclude that a selection bias may partially explain the high rate of response found in our study. Although the main clinical and virologic characteristics (age, prevalence of genotype 1) of our patients are those usually observed in nonresponders to combination therapy, in our series the prevalence of cirrhosis was only 16% and the majority of patients had a fibrosis stage lower than 3 according to the Ishak score, differing from Schiffman’s[14] study which included only patients with bridging fibrosis or cirrhosis. However, we observed a high SVR (30%) in patients with cirrhosis (stage 5 and 6) by the Ishak score and no significant difference in sustained response between patients with or without cirrhosis. We are therefore more prone to think that the higher antiviral efficacy found in our study is more likely linked to the addition of amantadine to combination therapy. In keeping with our results Maynard et al[33] demonstrated that 24% of non responders treated by triple therapy achieved an SVR of 16% of those treated by double therapy. The mechanism by which amantadine enhances the antiviral effect of interferon and ribavirin is unclear and remains largely speculative. It is well known that amantadine may act as an antiviral drug and its synergistic action in combination with IFN and RBV may function through the modulation of the host immune-response[36]. Although the mechanism underlying the potential synergism between these drugs is not clear, our results suggest that triple therapy, in particular when PegIFN is used, has to be considered as a valid option in nonresponders to combination therapy since it allows a sustained viral clearance in about one third of otherwise nonresponsive patients.
In keeping with the results obtained in treatment-naïve patients and in relapsers[1-4], PegIFN achieved a better response than standard interferon even though the latter was administered at a high daily dosage. Although the difference in SVR observed between the two treatment groups did not reach a statistical difference (P = 0.057), our results strongly suggest that in nonresponders, pegylated interferon should represent the first choice interferon in retreatment strategies. Our suggestion is further strengthened by the evidence of a lower incidence of therapy discontinuation in patients treated with PegIFN than in those treated with high dose standard interferon.
Baseline ALT value and undetectable serum HCV RNA at wk 12 of therapy were the variables independently associated with sustained response. Unexpectedly, HCV genotype did not emerge as an independent predictor of response by univariate analysis. In fact, in the present series, although genotype 1 patients responded less well than those with other genotypes, the difference did not maintain the significance at multivariate analysis. One possible explanation of this discrepancy is that the previous treatments may have selected patients in whom several and possibly new mechanisms of therapeutic resistance have emerged over time. In the present series the SVR in patients with genotype 1 was 22% (26% and 17% in those treated with PegIFN or standard IFN, respectively), a rate that is markedly higher than reported in other studies[14,37]. Interestingly, in treatment-naïve patients infected with genotype 1 the addition of amantadine to interferon therapy also led to an increased rate of SVR[38]. Although the problem remains of whether or not the retreatment of genotype 1 nonresponders is justified in terms of cost-efficacy[39], at the moment triple therapy including amantadine should be regarded as the most useful regimen to be proposed for these patients.
In the present study the addition of amantadine did not negatively affect the safety profile of combination therapy. In fact, triple therapy was found to be safe and well tolerated in the large majority of patients and the frequency and type of side effects did not differ from that observed in other series of nonresponders treated with standard or pegylated interferon plus ribavirin[1,2,40,41]. The higher rate of treatment withdrawal in patients treated with high dose standard recombinant interferon than in those receiving pegylated interferon parallels the results observed in treatment-naïve patients receiving pegylated interferon when compared to high dose induction therapy[42]. Interestingly, in our study, only two patients experienced depression severe enough to require treatment discontinuation. It is possible that the addition of amantadine, which is known to possess antidepressant properties[43,44], may have decreased the overall incidence of depression, which is often the cause of premature therapy withdrawal. This is another relevant aspect that, in association with the low cost of amantadine, supports the use of triple therapy in nonresponsive patients.
Although our study was not specifically designed to assess the predictive value of virologic response, some information can be drawn. Undetectable serum HCV RNA at wk 12 of therapy was the strongest independent predictor of SVR, in fact 50% of the patients who were HCV-RNA negative after 12 wk of treatment and 10% of those who were still positive, became sustained responders. In particular, the predictive negative value (90%) observed in our series at week 12 is slightly lower than that usually observed in treatment-naïve patients receiving combination therapy[45], suggesting that it may be more useful to wait until wk 24 before deciding whether to withdraw therapy. These findings further confirm that in this group of difficult-to-treat patients the variables and prognostic factors usually associated with response cannot be employed.
In conclusion, the present multicenter randomized study demonstrated that triple therapy, in particular employing pegylated-α-2a interferon plus ribavirin and amantadine, is an effective therapeutic option for patients nonresponsive to previous treatment with interferon plus ribavirin and can rescue at least one-fourth of these patients to sustained viral clearance. Although many questions remain regarding the role of amantadine in the treatment of HCV chronic hepatitis, these results, taken together with data from recent studies, provide encouragement for retreatment of nonresponders to combination therapy with triple therapy. Although our results need to be confirmed further in placebo-controlled studies, they seem to be particularly interesting when taking into account that addition of amantadine is low cost and safe and could offer hope to patients for whom no alternative therapies are available.
Pietro Corigliano, Department of Internal Medicine, Cantù Hospital, Abbiategrasso, Milano
Riccardo Bottelli, Department of Internal Medicine, Angera Hospital, Angera , Varese
Mario Strazzabosco, Bruno Paris, Gastroenterology Unit, Ospedali Riuniti, Bergamo
Angelo Rossini, Department of Internal Medicine, Spedali Civili, Brescia
Francesco Rocca, Moroni Marco, Severino Caprioli, Department of Internal Medicine and Department of Infectious Diseases, Azienda Ospedaliera di Circolo, Busto Arsizio, Como
Sergio Casati, Department of Internal Medicine, Cantù Hospital, Cantù , Como
Rossella Ferrini, Sant’Uboldo Hospital, Cernusco sul Naviglio, Milano
Giorgio Bellati, Alberto Colombo, Chiara Corradi, Department of Internal Medicine, Hepatology Unit, Sant’Anna Hospital, Como
Sergio Giorni, Davide Redaelli, Department of Internal Medicine, Civile Hospital, Garbagnate Milanese, Milano
Maria Antonietta Casiraghi, Department of Internal Medicine, Civile Hospital, Legnano
Giovanni Bolognini, Department of Internal Medicine, General Hospital, Lodi
GianPiero Benetti, Department of Internal Medicine, Liver Unit, Predabissi Hospital, Melegnano
Mauro Borzio, Department of Internal Medicine, Gastroenterology Unit, Fatebenefratelli Hospital, Milano
Silvia Fargion, Anna Ludovica Fracanzani, Alessandra Maraschi, Internal Medicine
Ospedale Maggiore Policlinico Mangiagalli e Regina Elena; Fondazione IRCCS, University of Milan, Milan
Giovanni Pinzello, Maria Vinci, Department of Internal Medicine, Gastroenterology Unit, Niguarda Hospital, Milano
Guido Croce, Andrea Capretti, Department of Internal Medicine, Gastroenterology Unit, San Carlo Borromeo Hospital, Milano
Antonietta Cargnel, Riccardo Giorni, Department of Infectious Diseases, Sacco Hospital, Milano
Massimo Pozzi, Alessandro Redaelli, Clinic of Internal Medicine, San Gerardo Hospital, Monza
Alberto Prada, Barbara Omazzi, Gastroenterology Unit, Uboldo Hospital, Rho, Milano
Aldo Autolitano, Department of Internal Medicine, Asilo Vittoria Hospital, Mortara
Paolo Del Poggio, Department of Internal Medicine, Hepatology, Caravaggio Hospital, Treviglio
S- Editor Pan BR L- Editor Alpini GD E- Editor Bi L
| 1. | Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Gonçales FL, Häussinger D, Diago M, Carosi G, Dhumeaux D. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4847] [Cited by in RCA: 4748] [Article Influence: 206.4] [Reference Citation Analysis (0)] |
| 2. | Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, Koury K, Ling M, Albrecht JK. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4736] [Cited by in RCA: 4558] [Article Influence: 189.9] [Reference Citation Analysis (0)] |
| 3. | Lindsay KL, Trepo C, Heintges T, Shiffman ML, Gordon SC, Hoefs JC, Schiff ER, Goodman ZD, Laughlin M, Yao R. A randomized, double-blind trial comparing pegylated interferon alfa-2b to interferon alfa-2b as initial treatment for chronic hepatitis C. Hepatology. 2001;34:395-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 469] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 4. | Zeuzem S, Feinman SV, Rasenack J, Heathcote EJ, Lai MY, Gane E, O'Grady J, Reichen J, Diago M, Lin A. Peginterferon alfa-2a in patients with chronic hepatitis C. N Engl J Med. 2000;343:1666-1672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 883] [Cited by in RCA: 851] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 5. | Keeffe EB. Chronic hepatitis C: management of treatment failures. Clin Gastroenterol Hepatol. 2005;3:S102-S105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Sethi A, Shiffman ML. Approach to the management of patients with chronic hepatitis C who failed to achieve sustained virologic response. Clin Liver Dis. 2005;9:453-71, vii-viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Gross JB. Non-responders to previous treatment for hepatitis C. Minerva Gastroenterol Dietol. 2005;51:47-54. [PubMed] |
| 8. | Cheng SJ, Bonis PA, Lau J, Pham NQ, Wong JB. Interferon and ribavirin for patients with chronic hepatitis C who did not respond to previous interferon therapy: a meta-analysis of controlled and uncontrolled trials. Hepatology. 2001;33:231-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 79] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Cummings KJ, Lee SM, West ES, Cid-Ruzafa J, Fein SG, Aoki Y, Sulkowski MS, Goodman SN. Interferon and ribavirin vs interferon alone in the re-treatment of chronic hepatitis C previously nonresponsive to interferon: A meta-analysis of randomized trials. JAMA. 2001;285:193-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 111] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 10. | Saracco G, Olivero A, Ciancio A, Carenzi S, Smedile A, Cariti G, Andreoni M, Orsi PG, Biglino A, Tabone M. A randomized 4-arm multicenter study of interferon alfa-2b plus ribavirin in the treatment of patients with chronic hepatitis C relapsing after interferon monotherapy. Hepatology. 2002;36:959-966. [PubMed] |
| 11. | Fargion S, Bruno S, Borzio M, Battezzati PM, Bissoli F, Ceriani R, Orlandi A, Maraschi A, Chiesa A, Morini L. Sustained response to combination therapy in patients with chronic hepatitis C who failed to respond to interferon. J Hepatol. 2003;38:499-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Davis GL, Esteban-Mur R, Rustgi V, Hoefs J, Gordon SC, Trepo C, Shiffman ML, Zeuzem S, Craxi A, Ling MH. Interferon alfa-2b alone or in combination with ribavirin for the treatment of relapse of chronic hepatitis C. International Hepatitis Interventional Therapy Group. N Engl J Med. 1998;339:1493-1499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 927] [Cited by in RCA: 895] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 13. | Hasan F, Al-Khaldi J, Asker H, Al-Ajmi M, Owayed S, Varghese R, Siddique I, Al-Nakib B. Peginterferon alpha-2b plus ribavirin with or without amantadine [correction of amantidine] for the treatment of non-responders to standard interferon and ribavirin. Antivir Ther. 2004;9:499-503. [PubMed] |
| 14. | Shiffman ML, Di Bisceglie AM, Lindsay KL, Morishima C, Wright EC, Everson GT, Lok AS, Morgan TR, Bonkovsky HL, Lee WM. Peginterferon alfa-2a and ribavirin in patients with chronic hepatitis C who have failed prior treatment. Gastroenterology. 2004;126:1015-1023; discussion 947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 343] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 15. | Jacobson IM, Gonzalez SA, Ahmed F, Lebovics E, Min AD, Bodenheimer HC Jr, Esposito SP, Brown RS Jr, Bräu N, Klion FM. A randomized trial of pegylated interferon alpha-2b plus ribavirin in the retreatment of chronic hepatitis C. Am J Gastroenterol. 2005;100:2453-2462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 87] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 16. | Marrache F, Consigny Y, Ripault MP, Cazals-Hatem D, Martinot M, Boyer N, Degott C, Valla D, Marcellin P. Safety and efficacy of peginterferon plus ribavirin in patients with chronic hepatitis C and bridging fibrosis or cirrhosis. J Viral Hepat. 2005;12:421-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Krawitt EL, Ashikaga T, Gordon SR, Ferrentino N, Ray MA, Lidofsky SD. Peginterferon alfa-2b and ribavirin for treatment-refractory chronic hepatitis C. J Hepatol. 2005;43:243-249. [RCA] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Wandinger KP, Hagenah JM, Klüter H, Rothermundt M, Peters M, Vieregge P. Effects of amantadine treatment on in vitro production of interleukin-2 in de-novo patients with idiopathic Parkinson's disease. J Neuroimmunol. 1999;98:214-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Skehel JJ, Hay AJ, Armstrong JA. On the mechanism of inhibition of influenza virus replication by amantadine hydrochloride. J Gen Virol. 1978;38:97-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 93] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Jubin R, Murray MG, Howe AY, Butkiewicz N, Hong Z, Lau JY. Amantadine and rimantadine have no direct inhibitory effects against hepatitis C viral protease, helicase, ATPase, polymerase, and internal ribosomal entry site-mediated translation. J Infect Dis. 2000;181:331-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Koff WC, Elm JL Jr, Halstead SB. Inhibition of dengue virus replication by amantadine hydrochloride. Antimicrob Agents Chemother. 1980;18:125-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Deltenre P, Henrion J, Canva V, Dharancy S, Texier F, Louvet A, De Maeght S, Paris JC, Mathurin P. Evaluation of amantadine in chronic hepatitis C: a meta-analysis. J Hepatol. 2004;41:462-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 52] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 23. | Engler S, Flechtenmacher C, Wiedemann KH, Gugler R, Stremmel W, Kallinowski B. Interferon alfa2a induction therapy in combination with ribavirin and amantadine for the treatment of naive patients with chronic HCV infection. J Viral Hepat. 2004;11:60-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Thuluvath PJ, Maheshwari A, Mehdi J, Fairbanks KD, Wu LL, Gelrud LG, Ryan MJ, Anania FA, Lobis IF, Black M. Randomised, double blind, placebo controlled trial of interferon, ribavirin, and amantadine versus interferon, ribavirin, and placebo in treatment naïve patients with chronic hepatitis C. Gut. 2004;53:130-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Brillanti S, Levantesi F, Masi L, Foli M, Bolondi L. Triple antiviral therapy as a new option for patients with interferon nonresponsive chronic hepatitis C. Hepatology. 2000;32:630-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 90] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 26. | Adinolfi LE, Utili R, Tonziello A, Ruggiero G. Effects of alpha interferon induction plus ribavirin with or without amantadine in the treatment of interferon non-responsive chronic hepatitis C: a randomised trial. Gut. 2003;52:701-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Di Bisceglie AM, Thompson J, Smith-Wilkaitis N, Brunt EM, Bacon BR. Combination of interferon and ribavirin in chronic hepatitis C: re-treatment of nonresponders to interferon. Hepatology. 2001;33:704-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 80] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 28. | Teuber G, Pascu M, Berg T, Lafrenz M, Pausch J, Kullmann F, Ramadori G, Arnold R, Weidenbach H, Musch E. Randomized, controlled trial with IFN-alpha combined with ribavirin with and without amantadine sulphate in non-responders with chronic hepatitis C. J Hepatol. 2003;39:606-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 29. | Younossi ZM, Mullen KD, Hodnick S, Barnes DS, Carey WD, McCullough AC, Easley K, Gramlich T, Liebermann BY. Triple combination of interferon alpha-2b, ribavirin, and amantadine for treatment of chronic hepatitis C. J Clin Gastroenterol. 2003;36:427-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 30. | Stauber RE, Hofer H, Hackl F, Schütze K, Datz C, Hegenbarth K, Jessner W, Steindl-Munda P, Peter F. Retreatment of patients with chronic hepatitis C not responding to interferon/ribavirin combination therapy with daily interferon plus ribavirin plus amantadine. Wien Klin Wochenschr. 2004;116:530-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 31. | Thuluvath PJ, Pande H, Maygers J. Combination therapy with interferon-alpha(2b), ribavirin, and amantadine in chronic hepatitis C nonresponders to interferon and ribavirin. Dig Dis Sci. 2003;48:594-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Oguz D, Cicek B, Filik L, Odemis B, Kilic M, Altintas E, Zengin N, Altiparmak E. Effect of interferon and ribavirin combined with amantadine in interferon and ribavirin non-responder patients with chronic hepatitis C (genotype 1). World J Gastroenterol. 2005;11:580-583. [PubMed] |
| 33. | Maynard M, Pradat P, Bailly F, Rozier F, Nemoz C, Si Ahmed SN, Adeleine P, Trépo C. Amantadine triple therapy for non-responder hepatitis C patients. Clues for controversies (ANRS HC 03 BITRI). J Hepatol. 2006;44:484-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 34. | Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween RN. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3521] [Cited by in RCA: 3782] [Article Influence: 126.1] [Reference Citation Analysis (1)] |
| 35. | Zilly M, Lingenauber C, Desch S, Väth T, Klinker H, Langmann P. Triple antiviral re-therapy for chronic hepatitis C with interferon-alpha, ribavirin and amantadine in nonresponders to interferon-alpha and ribavirin. Eur J Med Res. 2002;7:149-154. [PubMed] |
| 36. | Martín J, Navas S, Fernández M, Rico M, Pardo M, Quiroga JA, Zahm F, Carreño V. In vitro effect of amantadine and interferon alpha-2a on hepatitis C virus markers in cultured peripheral blood mononuclear cells from hepatitis C virus-infected patients. Antiviral Res. 1999;42:59-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 37. | Cammà C, Bruno S, Schepis F, Lo Iacono O, Andreone P, Gramenzi AG, Mangia A, Andriulli A, Puoti M, Spadaro A. Retreatment with interferon plus ribavirin of chronic hepatitis C non-responders to interferon monotherapy: a meta-analysis of individual patient data. Gut. 2002;51:864-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 38. | Mangia A, Leandro G, Helbling B, Renner EL, Tabone M, Sidoli L, Caronia S, Foster GR, Zeuzem S, Berg T. Combination therapy with amantadine and interferon in naïve patients with chronic hepatitis C: meta-analysis of individual patient data from six clinical trials. J Hepatol. 2004;40:478-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 39. | Ishida H, Inoue Y, Wong JB, Okita K. Cost-effectiveness of ribavirin plus interferon alpha-2b for either interferon relapsers or non-responders in chronic hepatitis C: a Japanese trial. Hepatol Res. 2004;28:125-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 40. | Reddy KR, Wright TL, Pockros PJ, Shiffman M, Everson G, Reindollar R, Fried MW, Purdum PP 3rd, Jensen D, Smith C. Efficacy and safety of pegylated (40-kd) interferon alpha-2a compared with interferon alpha-2a in noncirrhotic patients with chronic hepatitis C. Hepatology. 2001;33:433-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 261] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 41. | Heathcote EJ, Shiffman ML, Cooksley WG, Dusheiko GM, Lee SS, Balart L, Reindollar R, Reddy RK, Wright TL, Lin A. Peginterferon alfa-2a in patients with chronic hepatitis C and cirrhosis. N Engl J Med. 2000;343:1673-1680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 657] [Cited by in RCA: 605] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 42. | Bruno S, Cammà C, Di Marco V, Rumi M, Vinci M, Camozzi M, Rebucci C, Di Bona D, Colombo M, Craxì A. Peginterferon alfa-2b plus ribavirin for naïve patients with genotype 1 chronic hepatitis C: a randomized controlled trial. J Hepatol. 2004;41:474-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 80] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 43. | Huber TJ, Dietrich DE, Emrich HM. Possible use of amantadine in depression. Pharmacopsychiatry. 1999;32:47-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 61] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 44. | Bacosi M, Russo F, D'innocenzo S, Santolamazza M, Miglioresi L, Ursitti A, De Angelis A, Patrizi F, Ricci GL. Amantadine and interferon in the combined treatment of hepatitis C virus in elderly patients. Hepatol Res. 2002;22:231-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 45. | Trimoulet P, de Lédinghen V, Foucher J, Castéra L, Fleury H, Couzigou P. Predictive value of early HCV RNA quantitation for sustained response in nonresponders receiving daily interferon and ribavirin therapy. J Med Virol. 2004;72:46-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |