Published online Jan 21, 2006. doi: 10.3748/wjg.v12.i3.493
Revised: July 28, 2005
Accepted: August 3, 2005
Published online: January 21, 2006
The introduction of transjugular intrahepatic portal-systemic stent-shunt (TIPSS) has been a major breakthrough in the treatment of portal hypertension, which has evolved to a large extent, into a routine procedure. A 21-year-old male patient with progressive graft fibrosis/cirrhosis requiring TIPSS for variceal hemorrhage in the esophagus due to portal hypertension was unresponsive to conventional measures two years after living related liver transplantation (LDLT). Subsequently, variceal hemorrhage was controlled, however, liver function decreased dramatically with consecutive multi organ failure. CT scan revealed substantial necrosis in the liver. The patient underwent successful “high urgent” cadaveric liver transplantation and was discharged on postoperative d 20 in a stable condition.
- Citation: Schemmer P, Radeleff B, Flechtenmacher C, Mehrabi A, Richter GM, Büchler MW, Schmidt J. TIPSS for variceal hemorrhage after living related liver transplantation: A dangerous indication. World J Gastroenterol 2006; 12(3): 493-495
- URL: https://www.wjgnet.com/1007-9327/full/v12/i3/493.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i3.493
Transjugular intrahepatic portal-systemic stent-shunt (TIPSS) is defined as artificial shunts between branches of the portal vein and the systemic circulation within the liver parenchyma, and is comparable to a surgical H-shunt with partial decompression of portal hypertension. The first TIPSS has been performed by Rösch et al[1] in 1969 in dogs. After the stage had been set for the implantation of stent-shunts into human beings, the first TIPSS was performed on a 49-year-old male patient with liver cirrhosis and bleeding from esophageal varices due to portal hypertension[2]. Since then this procedure has rapidly progressed to a well defined and established standard procedure for a wide variety of liver disorders, i.e., TIPSS is preferred for acute or recurrent variceal hemorrhage refractory to medical treatment, endoscopic sclerotherapy or banding[3]. Since TIPSS enables pre-transplant patients to recover from bleeding episodes and improves their general status before a graft becomes available, this procedure forms a bridge to liver transplantation[4]. The first 30-d mortality after TIPSS (up to 11%) is usually due to liver failure, acute respiratory distress syndrome, sepsis, recurrent hemorrhage, or right heart or multi organ failure[4]. Here we report a case of liver necrosis with subsequent organ failure making it necessary for high urgency (HU) cadaveric liver transplantation following TIPSS for conventionally uncontrolled variceal hemorrhage after living related liver transplantation (LDLT).
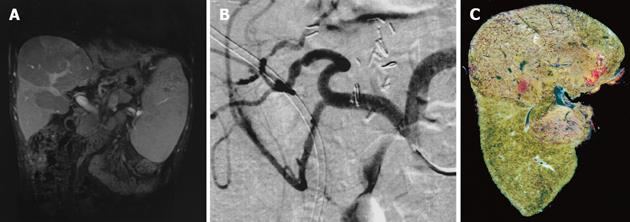
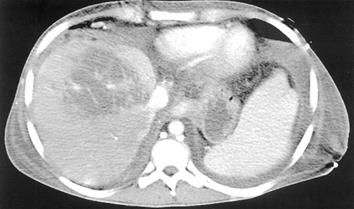
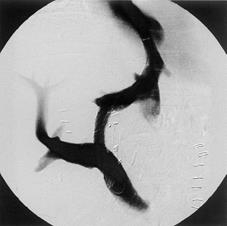
A 21-year-old male patient underwent LDLT with biliodigestive anastomosis because of liver cirrhosis due to hereditary tyrosinemia type I and hepatocellular carcinoma (T3N0M0). After an uneventful transplantation, the postoperative course was further complicated by a bilioma followed by both a biliocutaneous fistula and chronic cholangitis. Two years after LDLT, the patient was referred to our institution and presented with progressive cholestasis, all signs of portal hypertension (i.e., varicose veins of the esophagus and hypersplenism) while graft function was still adequate, classified as Child-Pugh A. Post-contrast sequences of MRI originally performed to identify the total extent of the biliocutaneous fistula, showed perfusion of the liver was inhomogeneous with regular appearance of the intra- and extrahepatic portal venous branches, inferior vena cava (IVC) and hepatic artery (Figure 1A). For a complete examination of the patient’s preoperative status, a celiac-mesenteric angiography was performed which identified a stenosis of the arterial anastomosis, considered as hemodynamically irrelevant (Figure 1B). Revision of the biliodigestive anastomosis was performed and a y-Roux hepatojejunostomy after adhesiolysis was established to close the biliocutaneous fistula, which maintained cholangitis. Histology of the intraoperative biopsy revealed severe fibrosis associated with secondary biliary cirrhosis of the transplant. After surgery the patient recovered quickly; however, on postoperative d 10 recurrent variceal hemorrhage unresponsive to conventional measures, including endoscopic therapy, required TIPSS since surgery was not feasible at that time. TIPSS was performed in a standard procedure with an uncoated 8/39 Corinthian-Stent, which decreased the portacaval pressure gradient from 23 mmHg (measured after a 8 mm tract was established) to 8 mmHg at the end of the procedure (Figure 2). Subsequently, variceal hemorrhage in the esophagus stopped, however, serum transaminases, bilirubin and ammonia increased, while liver function decreased dramatically with fulminant consecutive multi organ failure within 3 postoperative days. At that time, a spiral-CT scan revealed both substantial disturbances of intrahepatic perfusion and necrosis, while TIPSS, extrahepatic portal vein, hepatic artery and IVC displayed normal (Figures 2 and 3). One day later, the patient underwent successful (HU) orthotopic cadaveric re-transplantation of the liver and was discharged in a stable condition thereafter on postoperative d 20.
Biliary complications after LDLT are observed in 15-40% of patients, which can increase the risk of developing secondary biliary cirrhosis and all signs of portal hypertension (i.e. variceal bleeding)[5]. The significant decrease of portal flow to the liver after TIPSS, due to portal decompression, may result in decreased liver function. An increase in hepatic arterial flow, however, maintains sufficient liver function in most patients[9]. The latter is inadequate in a small number of patients and TIPSS results in liver failure[4]. Further, cirrhotic patients have a cirrhotic cardiomyopathy. This is important since TIPSS shunts a large blood volume back to the right heart and may impair cardiac function.
Till date most information of both arterial and portal venous hemodynamic in the liver are based on indirect scintigraphic or invasive electromagnetic measurement of blood flow during portosystemic shunt surgery. Burchell et al[6] demonstrated in their early work an increased blood flow in the hepatic artery after porto-caval shunting. Since no flow measurements were performed intravascularly, precise information on complex flow changes over time (i.e., detection of mechanisms of arterio-portal compensation) were impossible[6]. In the late sixties Hanson[7] and Lutz[8] hypothesized that both dilatation and contraction of the intrahepatic arterioles, which occur immediately after liver- or shunt-operations, can be defined as regulatory reflex-mediated responses to changes of pressure in the hepatic sinusoids. Richter et al[9] later demonstrated online the dynamic changes of arterial and portal blood flow during partial porto-systemic decompression with TIPSS. Real-time monitoring of the arterial flow with endoluminal catheters revealed that arterial blood flow increased significantly and momentarily while portal venous flow decreased; however, total liver perfusion after TIPSS maintained steady in their studies. Since the fraction of arterial perfusion was increased after TIPSS, both pressure of portal venous perfusion and portal flow in hepatic sinusoids decreased. Reduced portal venous inflow in hepatic capillaries was compensated by an increased arterial perfusion. Since these changes in hepatic flow were immediately present in all the patients after TIPSS, underlying regulatory mechanisms that maintain a constant sinusoidal liver perfusion are most likely based on reflexes.
How can it be explained that major disturbances in hepatic circulation with subsequent liver necrosis and deterioration of liver function occurred after TIPSS in the case described above Several factors most likely contributed to pathology: One major factor was the initially compensated stenosis of the hepatic artery, diagnosed with a celiac-mesenteric angiography (Figure 1B), which most likely prevented an increase in the arterial flow reserve to compensate for a decreased portal flow after TIPSS. Moreover, chronic inflammation (cholangitis) and liver cirrhosis may have also factored into the reduction of possible regulatory mechanisms stated above that compensated for reduced portal flow[10,11]. Therefore, severe dysfunction of the liver due to disturbances in hepatic perfusion, with subsequent necrosis of liver tissue, complicated treatment after TIPSS (Figures 1C and 3). In such cases, TIPSS should be indicated with great caution in centers where the salvage therapy, high urgent liver transplantation, can be performed.
S- Editor Guo SY L- Editor Elsevier HK E- Editor Kong LH
| 1. | Rösch J, Hanafee WN, Snow H. Transjugular portal venography and radiologic portacaval shunt: an experimental study. Radiology. 1969;92:1112-1114. [PubMed] |
| 2. | Richter GM, Palmaz JC, Nöldge G, Rössle M, Siegerstetter V, Franke M, Wenz W. [The transjugular intrahepatic portosystemic stent-shunt. A new nonsurgical percutaneous method]. Radiologe. 1989;29:406-411. [PubMed] |
| 3. | Rösch J, Keller FS. Transjugular intrahepatic portosystemic shunt: present status, comparison with endoscopic therapy and shunt surgery, and future prospectives. World J Surg. 2001;25:337-45; discussion 345-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 68] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Barton RE, Rösch J, Saxon RR, Lakin PC, Petersen BD, Keller FS. TIPS: short and long-term results: a survey of 1750 patients. Sem Interv Radiol. 1995;12:364-369. [RCA] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Shiffman ML, Brown RS, Olthoff KM, Everson G, Miller C, Siegler M, Hoofnagle JH. Living donor liver transplantation: summary of a conference at The National Institutes of Health. Liver Transpl. 2002;8:174-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 102] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 6. | Burchell AR, Moreno AH, Panke WF, Nealon TF. Hepatic artery flow improvement after portacaval shunt: a single hemodynamic clinical correlate. Ann Surg. 1976;184:289-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 74] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Hanson KM, Johnson PC. Local control of hepatic arterial and portal venous flow in the dog. Am J Physiol. 1966;211:712-720. [PubMed] |
| 8. | Lutz J, Peiper U, Bauereisen E. [Appearance and size of veno-vasomotor reactions in liver circulation]. Pflugers Arch Gesamte Physiol Menschen Tiere. 1968;299:311-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 14] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Richter GM, Brado M, Simon C, Mädler U, Radeleff B, Roeren T, Sauer P, Kauffmann GW. [Changes in liver perfusion caused by transjugular intrahepatic stent shunt (TIPSS)]. Zentralbl Chir. 1997;122:108-116. [PubMed] |
| 10. | Bader TR, Braga L, Beavers KL, Semelka RC. MR imaging findings of infectious cholangitis. Magn Reson Imaging. 2001;19:781-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Revelon G, Rashid A, Kawamoto S, Bluemke DA. Primary sclerosing cholangitis: MR imaging findings with pathologic correlation. AJR Am J Roentgenol. 1999;173:1037-1042. [PubMed] |