Published online Jan 21, 2006. doi: 10.3748/wjg.v12.i3.473
Revised: June 8, 2005
Accepted: June 24, 2005
Published online: January 21, 2006
AIM: To study whether heat-shocked tumor cells could enhance the effect of tumor cell lysate-pulsed dendritic cells (DCs) in evoking anti-tumor immune response in vivo.
METHODS: Mouse undifferentiated colon cancer cells (CT-26) were heated at 42 °C for 1 h and then frozen-thawed. The bone marrow-derived DCs pulsed with heat-shocked CT-26 cell lysate (HSCT-26 DCs) were recruited to immunize syngeneic naïve BALB/c mice. The cytotoxic activity of tumor specific cytotoxic T lymphocytes (CTLs) in mouse spleen was evaluated by IFN-enzyme-linked immunospot (ELISpot) and LDH release assay. The immunoprophylactic effects induced by HSCT-26 DCs in mouse colon cancer model were compared to those induced by single CT-26 cell lysate-pulsed DCs (CT-26 DCs) on tumor volume, peritoneal metastasis and survival time of the mice.
RESULTS: Heat-treated CT-26 cells showed a higher hsp70 protein expression. Heat-shocked CT-26 cell lysate pulsing elevated the co-stimulatory and MHC-II molecule expression of bone marrow-derived DCs as well as interleukin-12 p70 secretion. The IFN-γ secreting CTLs induced by HSCT-26 DCs were significantly more than those induced by CT-26 DCs (P = 0.002). The former CTLs’ specific cytotoxic activity was higher than the latter CTLs’ at a serial E/T ratio of 10:1, 20:1, and 40:1. Mouse colon cancer model showed that the tumor volume of HSCT-26 DC vaccination group was smaller than that of CT-26 DC vaccination group on tumor volume though there was no statistical difference between them (24 mm3 vs 8 mm3, P = 0.480). The median survival time of mice immunized with HSCT-26 DCs was longer than that of those immunized with CT-26 DCs (57 d vs 43 d, P = 0.0384).
CONCLUSION: Heat-shocked tumor cell lysate-pulsed DCs can evoke anti-tumor immune response in vivo effectively and serve as a novel DC-based tumor vaccine.
-
Citation: Qiu J, Li GW, Sui YF, Song HP, Si SY, Ge W. Heat-shocked tumor cell lysate-pulsed dendritic cells induce effective anti-tumor immune response
in vivo . World J Gastroenterol 2006; 12(3): 473-478 - URL: https://www.wjgnet.com/1007-9327/full/v12/i3/473.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i3.473
Dendritic cells (DCs) are the most potent antigen-presenting cells (APCs) mediating effective immune effects in vitro and in vivo[1,2]. DCs-based tumor vaccines have been recruited to prevent postoperative recurrence and metastasis of malignant tumors[3,4]. During the development of this approach, distinct methods have been attempted to enhance the effect of DC tumor vaccine in evoking tumor rejection response.
Heat shock protein (HSP), the molecule chaperon in cells, binds to the antigenic peptides and guides them to accurate folding, transporting, and conjugating to major histocompatibility complex (MHC) molecules[5]. Referring to the current knowledge of HSPs, it acts as chaperone peptides including antigenic peptides, interacts with antigen presenting cells through a receptor, stimulates antigen presenting cells to secrete inflammatory cytokines and mediates maturation of DCs. It was reported that heating could enhance the immunogenicity of tumor cells, which is ascribed to HSPs[6]. Furthermore, vaccination with the lysate of heated tumor cells can result in effective tumor rejection in vivo[7]. In the present study, we used heat-shocked tumor cells to elicit HSP expression. We assumed that pulsing with these HSP-rich tumor cell lysate might enhance the effect of DCs to stimulate stronger anti-tumor response than pulsing with tumor cell lysates. Because hyperthermal treatment is a standardized manipulation in clinical practice of oncologic surgery, such a DC tumor vaccine is convenient to be prepared. We examined the specific CTL response induced by heat-shocked tumor cell lysate-pulsed DCs in naïve mice and further evaluated its immunoprophylactic effects in mouse colon cancer model.
Six to eight-week-old female BALB/c mice were purchased from Laboratory Animal Research Center (LARC) of the Fourth Military Medical University (FMMU, Xi’an) and housed under pathogen-free conditions. All experiments involving the use of mice were performed in accordance with the protocols approved by LARC. CT26 is a carcinogen-induced undifferentiated colon adenocarcinoma cell line of BALB/c mice. Cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM, Gibco) containing 10% heat-inactivated fetal bovine serum (FBS; Sijiqin Biotech, Hangzhou), 100 IU/mL penicillin and 100 µg/mL streptomycin at 37 °C in an atmosphere containing 50 mL/L CO2 and passaged every 2 d.
CT-26 cells at 90% confluence were heated in 42 °C water bath for 1 h followed by recovering for 2 h at 37 °C in an atmosphere containing 50 mL/L CO2. Cells were then digested by 0.02% trypsin and washed twice with PBS. After being enumerated and re-suspended in PBS at 1×106 cells/mL, CT-26 cells were frozen in liquid nitrogen for 10 min and then thawed thrice at 4 °C. The frozen-thawed resultants were centrifuged at 12 000 g for 15 min and the supernatant was preserved as tumor cell lysate at -80 °C.
Equivalent protein samples were subjected to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Then, the separated proteins were transferred onto nitrocellulose (NC) membranes. Membranes were blocked with 5% non-fat milk in TBST (20 mmol/L Tris-HCl, pH 7.4, 150 mmol/L NaCl, and 0.05% Tween 20) for 2 h and incubated for 1 h at room temperature with hsp70 mAb (H5147, Sigma) diluted in TBST. After washing, membranes were incubated with horseradish peroxidase conjugated goat-anti-rabbit IgG (Boxter) diluted 1:400 in TBST at room temperature for 1 h. Detection was performed using the DAB detection system.
Bone marrow-derived DCs were generated as described by Lutz et al[8] with minor modifications. Briefly, 1×106 cells/mL erythrocyte-depleted mouse bone marrow cells from flushed marrow cavities were cultured in complete medium (CM) with 20 ng/mL recombinant mouse GM-CSF (PeproTech, Rocky Hill, NJ, USA) in 10-cm tissue culture dishes at 37 °C in an atmosphere containing 50 mL/L CO2. On d 3, 5, and 7, respectively, half media were removed and centrifuged for 5 min at 1 500 r/min, the collected cells were resuspended in the same volume of fresh CM and replenished to original plates. The frozen-thawed tumor lysate was added to the DC culture systems on d 7 at a ratio of five DC equivalents to one tumor cell (i.e., 5:1) and incubated at 37 °C in an atmosphere containing 50 mL/L CO2. After 48 h of incubation, non-adherent cells including DCs were harvested by gentle pipetting. DCs were enumerated by FACS (FACScan, Becton Dickinson) analysis through staining with PE anti-mouse CD11c Ab (N418, hamster IgG, Biolegend). The co-stimulatory and MHC-II molecules were analyzed by staining with FITC anti-mouse I-A/I-E (m5/114.15.2, ratIgG2b, Biolegend) and FITC anti-mouse CD86 (B7-2, PO3, ratIgG2b, Biolegend). The corresponding labeled isotypes served as the controls. For further vaccination, DCs were washed twice, enumerated and resuspended in PBS at 5×106/mL.
Following the protocol of mIL-12 p70 ELISA Ready-SET-Go kit (Ebioscience, San Diego, CA, USA), plates (NUNC Maxisorp) were pre-coated with capture antibody (clone C18.2) overnight at 4 °C and blocked at room temperature for 1 h. After being washed, 100 µL/well of mIL-12 p70 standard (8 pg-1 024 pg/mL at twofold serial dilutions) or 100 µL/well of samples was added to the appropriate wells and incubated at room temperature for 2 h. Wells were aspirated and washed, then 100 µL/well detection antibody (clone C17.8) was added and incubated at room temperature for 1 h. After being washed, 100 µL avidin-HRP was added and incubated at room temperature for 30 min. Plates were washed thoroughly and 100 µL/well of substrate solution was added. After being incubated at room temperature for 15 min, 50 µL of stop solution was added to each well. The plates were read. Data represented the value of 450 nm subtracted the value of 570 nm. All assays were performed in triplicate.
BALB/c mice were immunized with DC vaccine through tail-vein on d 0 and 7 respectively at the same dose of 5×105 DCs (100 µL). Each treatment group contained not less than 15 mice. Seven days after the second immunization, three mice of each group were killed and the spleens were taken to perform the ELISpot and cytotoxic assays. The remaining mice were anesthetized with 0.75 mg of sodium pentobarbital. Colonic cancer inoculation was performed as follows. In brief, a vertical midline incision was made and the cecum was exposed. Upon visualization, 1×105 CT-26 cells (50 µL) were injected into subserosa using a 30-gauge needle (Becton Dickinson) and 1 mL syringe. The midline incision was closed with a running suture. Fourteen days later, not less than five mice of each group were killed, the weight of the colon tumors was measured and the peritoneal metastases were checked. The remaining mice were fed to observe the tumor-bearing survival time. The end point of observation was selected when all the mice of any of the two teams were dead.
The murine interferon-gamma ELISpot kit (Diaclone, France) was used to determine tumor-specific IFN-γ secreting T cells[9]. The 96-well filtration plates (Nunc) were coated with 100 µL capture antibody (clone DB1). After an overnight incubation at 4 °C, the wells were washed and blocked with 2% dry skimmed milk in PBS. Splenocytes (1×106/well) isolated from the mice were added to the wells and incubated at 37 °C in an atmosphere containing 50 mL/L CO2 for 20 h with target cells (5×104/well). Plates were washed and then incubated with 100 µL biotinylated detection antibody (polyclonal) at 37 °C for 90 min. After the removal of unbound antibodies, 100 µL avidin-alkaline phosphatase was added and plates were incubated for 1 h at 37 °C. After washing, spots were developed by adding 100 µL of ready-to-use BCIP/NBT buffer and incubated at room temperature for spot formation. The spots were scanned and counted.
Specific cytolytic activities of murine spleen cells were determined by LDH assay. The CT-26 cells were used as target cells. Effector and target cells were mixed at the E/T ratio of 20:1 at 0.2 mL/well in 96-well round-bottomed plates (Nunc). After incubation for 4 h, cells were centrifuged at 250 r/min for 5 min and the cell-free supernatant was collected for LDH assay using CytoTox96 (Promega, Madison, WI, USA). The percentage of specific LDH release was calculated by the following formula: % cytotoxicity = [(experimental LDH release)-(spontaneous LDH release by effector and target cells)/(maximum LDH release)×(spontaneous LDH release)]×100. For the controls, the target cells were incubated either in culture medium alone to determine spontaneous release or in a mixture of 2% Triton X-100 to define the maximum LDH release. The spontaneous release was always <10% of the maximum release. All assays were performed in triplicate.
Each group included at least five mice. All experiments were carried out thrice. Data were expressed as mean ± SD. Tumor volume and immune cell yield data were analyzed by two-way analysis of variance (ANOVA). All analyses were conducted with SPSS8.0 software. P < 0.05 was considered statistically significant.
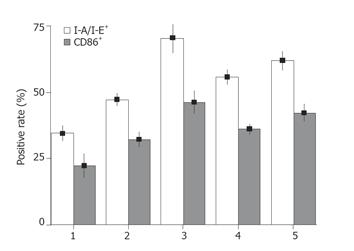
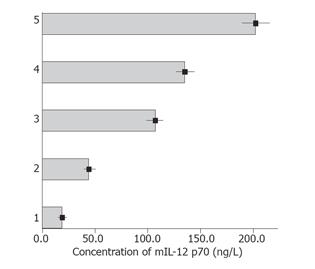
To evaluate the hsp70 protein expression in CT-26 cells, we performed Western blot analysis using an anti-hsp70 mAb. hsp70 protein in CT-26 cells increased after being heated for 30 min and peaked at 1 h (Figure 1). Therefore, heating at 42 °C for 1 h was selected as a standard treatment for heat shocking in the following experiment. To determine the quantity of DCs in culture system, we analyzed the surface molecules of harvested cells by flow cytometry. About 65% of the harvest cells in culture system on d 7 were CD11c positive and increased to 75% or more on d 9. DCs pulsed with HSCT-26 lysate manifested higher CD86 and I-A/I-E expression than pulsed with CT-26 lysate (Figure 2). We also measured the mIL-12 p70 concentration in the supernatant of different DC culture systems by ELISA. HSCT-26 lysate-pulsed DCs showed a higher level of mIL-12 p70 than either CT-26 lysate-pulsed or unpulsed DCs on d 9 (Figure 3).
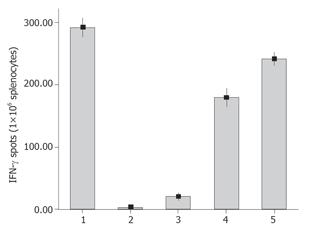
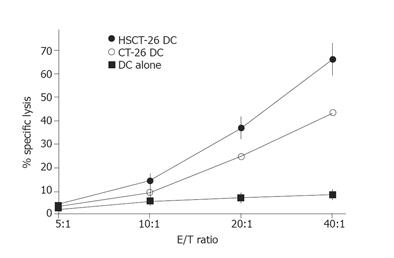
To investigate the ability of HSCT-26 lysate-pulsed DCs to induce CT-26 specific CTLs in naïve syngeneic BALB/c mice, we examined the CT-26 specific IFN-γ-producing T cells in splenocytes derived from immunized and control mice by ELISpot assay. As shown in Figure 4, after being restimulated with mitomycin C-treated CT-26 cells at an E:T ratio of 20:1, the CT-26 specific IFN-γ secreting T cells in HSCT-26 DCs-immunized mice were significantly more than those in CT-26 DCs-immunized mice (P = 0.002). Then we evaluated the CT-26 specific cytolytic activity of splenocytes stimulated with different DC tumor vaccines. As shown in Figure 5, splenocytes from mice that received HSCT-26 DC vaccination displayed significantly stronger cytolytic activity against CT-26 cells at ratios 10:1, 20:1, and 40:1 of effector cells to target cells.
We examined whether heat-shocked CT-26 cells lysate-pulsed DCs could display enhanced immunoprophylactic potential in mouse colon cancer model. After being vaccinated twice at an interval of 7 d, BALB/c mice were inoculated with CT-26 cells into cecum through open surgery. Fourteen days after the inoculation, autopsies were performed to check the growth and metastasis of colon tumor. The mice that received CT-26 DC vaccination or single DC vaccination had colon tumor formation, whereas one of the six mice that received HSCT-26 DC vaccination was tumor free. The tumor volumes in single DC vaccination group, CT-26 DC vaccination group and HSCT-26 DC vaccination group were 107 ± 69, 24 ± 8, and 8 ± 7 mm3, respectively. ANOVA analysis showed significant differences in tumor volume among the three groups (P = 0.000), but there was no difference between HSCT-26 DC vaccination group and CT-26 DC vaccination group (P = 0.480). Neither HSCT-26 DC vaccination group nor CT-26 DC vaccination group had peritoneal metastasis. In contrast, 50% mice in single DC vaccination group (3/6) had tumor planting to adjacent peritoneum and intestine surface accompanied with a small quantity of bloody ascites. No hepatic metastasis was found in the mice of the three groups macroscopically during autopsy.
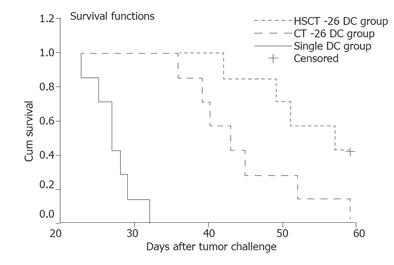
To investigate the tumor-bearing survival time, we observed immunized mice for at least 60 d. The median survival time of mice in the single DC vaccination group, CT-26 DC vaccination group and HSCT-26 DC vaccination group was 27.0, 43.0, and 57.0 d, respectively. Log-rank test showed that the median survival time was significantly different in the three groups of mice (P = 0.0001). Most importantly, the mice vaccinated with HSCT-26 DCs had a much longer survival time than those vaccinated with CT-26 DCs (P = 0.0384). The survival curves are shown in Figure 6.
Radical surgery is the standard treatment for most patients with advanced cancer. After resection of primary tumor and local drainage lymph nodes, the tumor burden of patients is released and the immune system may have a good chance for recovery. It is the critical time to commence adjuvant immunotherapy for preventing postoperative recurrence and metastasis. The prophylactic effects of this therapy last for a long time and can be augmented by revaccination.
The anti-tumor effect of tumor cell lysate-pulsed DCs was first reported in 1998[10] and has been utilized to treat malignant diseases including renal cancer, lymphoma, and colorectal cancer, etc.[11-13]. However, a misgiving of rising autoimmunity still exists as shown in animal models[14]. Current pilot clinical studies have not found clinical signs of autoimmune disease except for vitiligo and occurrence of auto antibodies. Reinhard et al[15] reported that vaccination with tumor lysate-pulsed DCs does not show a higher incidence of autoimmunity than vaccination with peptide-pulsed DCs. Tumor lysate is superior to other ways of DC pulsing and can elicit immunity for the entire array of TAAs of source tumors, circumventing the need of prior identification of TAAs from individual cancers. Enhancing the anti-tumor effect of whole tumor cell lysate-pulsed DCs promotes the use of this kind of tumor vaccine.
In our present study, tumor cells heated at 42 °C increased the expression of HSPs such as HSP70. Pulsed with such lysates of heat-shocked tumor cells, DCs showed higher expression of MHC-II and co-stimulatory molecules on their surface and secreted more mIL-12 P70. As we know, DC priming T lymphocytes require signals I and II. Signal I presents the antigenic epitopes introduced by MHC molecules. Signal II as the co-stimulatory molecules on DC surface, can improve epitopes of DCs by interacting with their respective ligands on T lymphocytes. Upregulated expression of MHC-II and co-stimulatory molecules on DC surface facilitates activation of T lymphocytes effectively. IL-12 is a cytokine leading to cell-mediated immune response (Th1 response) and is preferable for successful tumor immunotherapy[16]. Heat-induced HSPs may also serve as endogenous danger signals to drive DC maturation. Mature DCs obtain high viability and present antigenic epitopes to T lymphocytes through migrating to local drainage lymph nodes. Our result is in agreement with related reports[17]. DC activating process may be involved in NF-κB pathway[18].
It is believed that HSP-chaperoned TAAs represent the specific fingerprint of tumor cells. A gp96 receptor, CD91 on APC surface has been recently identified[19,20]. HSP-chaperoned antigen might target to APCs with the assistance of HSP receptors on these cells. HSP-peptide complexes can be internalized by APCs through receptor-mediated endocytosis[21]. HSP-mediated epitopes could go through endogenous antigen-processing pathways in the context of MHC-Imolecules[22]. DCs have an impressive ability to “cross-present” MHC class I-restricted peptide epitopes derived from exogenous proteins to CD8 T lymphocytes. In our study, compared to tumor cell lysate-pulsed DCs, immunization with heat-shocked tumor cell lysate-pulsed DCs might induce a large amount of specific CTLs in naïve mice in vivo. Furthermore, the induced CTLs exhibited a higher tumor specific cytotoxic activity. Immunized and challenged mouse model proved that vaccination of HSCT-26 DCs could prolong the survival time of tumor-bearing animals. All the results suggest that heating tumor cells can enhance the effect of whole tumor cell lysate-pulsed DCs to elicit specific anti-tumor response. We suppose that after taking up the HSP-chaperoned antigens, epitopes coming from HSCT-26 might be complexed with MHC class I molecules in endoplasmic reticulum and transported to the cell surface to priming CD8 T lymphocytes. Increased HSPs in tumor cells may serve as epitope chaperons and natural adjuvants in the process of DC antigen presentation, enhancing anti-tumor effects of DC vaccine.
In conclusion, heat-shocked tumor cell lysate-pulsed DCs can elicit effective anti-tumor effects in vivo, suppress the growth of colon cancer and eliminate occurrence of peritoneal metastasis. Using this tumor vaccine postoperatively may decrease the local recurrence and peritoneal metastasis of colon cancer and prolong the tumor-free survival time of the patients. The prophylactic effects of this DC vaccine on preventing liver metastasis of colon cancer should be further evaluated.
S- Editor Ma JY and Guo SY L- Editor Elsevier HK E- Editor Kong LH
| 1. | Banchereau J, Schuler-Thurner B, Palucka AK, Schuler G. Dendritic cells as vectors for therapy. Cell. 2001;106:271-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 298] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 2. | Whiteside TL, Odoux C. Dendritic cell biology and cancer therapy. Cancer Immunol Immunother. 2004;53:240-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 67] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 3. | Schuler G, Schuler-Thurner B, Steinman RM. The use of dendritic cells in cancer immunotherapy. Curr Opin Immunol. 2003;15:138-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 468] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 4. | Mocellin S, Rossi CR, Lise M, Nitti D. Colorectal cancer vaccines: principles, results, and perspectives. Gastroenterology. 2004;127:1821-1837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Audibert F. Adjuvants for vaccines, a quest. Int Immunopharmacol. 2003;3:1187-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Ménoret A, Patry Y, Burg C, Le Pendu J. Co-segregation of tumor immunogenicity with expression of inducible but not constitutive hsp70 in rat colon carcinomas. J Immunol. 1995;155:740-747. [PubMed] |
| 7. | Okamoto M, Tazawa K, Kawagoshi T, Maeda M, Honda T, Sakamoto T, Tsukada K. The combined effect against colon-26 cells of heat treatment and immunization with heat treated colon-26 tumour cell extract. Int J Hyperthermia. 2000;16:263-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Lutz MB, Kukutsch N, Ogilvie AL, Rössner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2380] [Cited by in RCA: 2515] [Article Influence: 96.7] [Reference Citation Analysis (0)] |
| 9. | Miyahira Y, Murata K, Rodriguez D, Rodriguez JR, Esteban M, Rodrigues MM, Zavala F. Quantification of antigen specific CD8+ T cells using an ELISPOT assay. J Immunol Methods. 1995;181:45-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 288] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 10. | Fields RC, Shimizu K, Mulé JJ. Murine dendritic cells pulsed with whole tumor lysates mediate potent antitumor immune responses in vitro and in vivo. Proc Natl Acad Sci U S A. 1998;95:9482-9487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 352] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 11. | Höltl L, Zelle-Rieser C, Gander H, Papesh C, Ramoner R, Bartsch G, Rogatsch H, Barsoum AL, Coggin JH, Thurnher M. Immunotherapy of metastatic renal cell carcinoma with tumor lysate-pulsed autologous dendritic cells. Clin Cancer Res. 2002;8:3369-3376. [PubMed] |
| 12. | Maier T, Tun-Kyi A, Tassis A, Jungius KP, Burg G, Dummer R, Nestle FO. Vaccination of patients with cutaneous T-cell lymphoma using intranodal injection of autologous tumor-lysate-pulsed dendritic cells. Blood. 2003;102:2338-2344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 98] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 13. | Rains N, Cannan RJ, Chen W, Stubbs RS. Development of a dendritic cell (DC)-based vaccine for patients with advanced colorectal cancer. Hepatogastroenterology. 2001;48:347-351. [PubMed] |
| 14. | Ludewig B, Ochsenbein AF, Odermatt B, Paulin D, Hengartner H, Zinkernagel RM. Immunotherapy with dendritic cells directed against tumor antigens shared with normal host cells results in severe autoimmune disease. J Exp Med. 2000;191:795-804. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 197] [Cited by in RCA: 189] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 15. | Reinhard G, Märten A, Kiske SM, Feil F, Bieber T, Schmidt-Wolf IG. Generation of dendritic cell-based vaccines for cancer therapy. Br J Cancer. 2002;86:1529-1533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Mashino K, Sadanaga N, Tanaka F, Ohta M, Yamaguchi H, Mori M. Effective strategy of dendritic cell-based immunotherapy for advanced tumor-bearing hosts: the critical role of Th1-dominant immunity. Mol Cancer Ther. 2002;1:785-794. [PubMed] |
| 17. | Kuppner MC, Gastpar R, Gelwer S, Nössner E, Ochmann O, Scharner A, Issels RD. The role of heat shock protein (hsp70) in dendritic cell maturation: hsp70 induces the maturation of immature dendritic cells but reduces DC differentiation from monocyte precursors. Eur J Immunol. 2001;31:1602-1609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 18. | Basu S, Binder RJ, Suto R, Anderson KM, Srivastava PK. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kappa B pathway. Int Immunol. 2000;12:1539-1546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 949] [Cited by in RCA: 920] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 19. | Wassenberg JJ, Dezfulian C, Nicchitta CV. Receptor mediated and fluid phase pathways for internalization of the ER Hsp90 chaperone GRP94 in murine macrophages. J Cell Sci. 1999;112:2167-2175. [PubMed] |
| 20. | Binder RJ, Han DK, Srivastava PK. CD91: a receptor for heat shock protein gp96. Nat Immunol. 2000;1:151-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 526] [Cited by in RCA: 489] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 21. | Srivastava PK, Amato RJ. Heat shock proteins: the 'Swiss Army Knife' vaccines against cancers and infectious agents. Vaccine. 2001;19:2590-2597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 112] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 22. | Singh-Jasuja H, Hilf N, Arnold-Schild D, Schild H. The role of heat shock proteins and their receptors in the activation of the immune system. Biol Chem. 2001;382:629-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 56] [Article Influence: 2.3] [Reference Citation Analysis (0)] |