Published online Jan 21, 2006. doi: 10.3748/wjg.v12.i3.420
Revised: June 28, 2005
Accepted: July 15, 2005
Published online: January 21, 2006
AIM: To investigate the action of genistein (GST), a broad spectrum tyrosine kinase inhibitor, on voltage-gated potassium channels in guinea pig proximal colon smooth muscle cells.
METHODS: Smooth muscle cells in guinea pig proximal colon were enzymatically isolated. Nystatin-perforated whole cell patch clamp technique was used to record potassium currents including fast transient outward current (IKto) and delayed rectifier current (IKdr), two of which were isolated pharmacologically with 10 mmol/L tetraethylammonium or 5 mmol/L 4-aminopyridine. Contamination of calcium-dependent potassium currents was minimized with no calcium and 0.2 mmol/L CdCl2 in an external solution.
RESULTS: GST (10-100 µmol/L) reversibly and dose-dependently reduced the peak amplitude of IKto with an IC50 value of 22.0±6.9 µmol/L. To a lesser extent, IKdr was also inhibited in both peak current and sustained current. GST could not totally block the outward potassium current as a fraction of the outward potassium current, which was insensitive to GST. GST had no effect on the steady-state activation (n = 6) and inactivation kinetics (n = 6) of IKto. Sodium orthovanadate (1 mmol/L), a potent inhibitor of tyrosine phosphatase, significantly inhibited GST-induced inhibition (P < 0.05).
CONCLUSION: GST can dose-dependently and reversibly block voltage-gated potassium channels in guinea pig proximal colon smooth muscle cells.
- Citation: Li SY, Huang BB, Ouyang S. Effect of genistein on voltage-gated potassium channels in guinea pig proximal colon smooth muscle cells. World J Gastroenterol 2006; 12(3): 420-425
- URL: https://www.wjgnet.com/1007-9327/full/v12/i3/420.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i3.420
Ion channels are targets of many intracellular signaling pathways, including protein phosphorylation and dephosphorylation. These processes can modify channel activity and dramatically alter the electrophysiological properties of both excitable and nonexcitable cells[1]. In addition to the extensive information available about the regulation of ion channels by serine-threonine kinases, an emerging body of evidence suggests that channels are also regulated by phosphorylation on tyrosine residues, such as ligand-gated channels AChR[2], NMDA receptor[3], potassium channels[4,5] as well as calcium channels[6,7]. GST, a specific inhibitor of tyrosine kinases, inhibits visceral smooth muscle contraction induced by tyrosine phosphatase inhibitor vanadate[8], angiotensin II[9] and carbachol[10] via tyrosine kinase-mediated process. Potassium channels, which are substrates for protein phosphorylation and dephosphorylation, control the contraction of gastrointestinal smooth muscles by setting resting potential and influencing slow waves and action potential configuration[11]. However, little is known of the modulation of potassium channels in gastrointestinal smooth muscle cells via tyrosine kinase pathway. This study was to investigate the effects and mechanisms of GST on voltage-gated potassium channels (Kv) in smooth muscle cells of guinea pig proximal colon.
Smooth muscle cells were enzymatically isolated using modified procedures as previously described[12]. Briefly, male guinea pigs (200-350 g) were killed by cervical dislocation and proximal colon about 2 cm aboral to cecum was rapidly excised. Under anatomical microscope, smooth muscle strips were dissected out, cut into small pieces and incubated for 30 min in low calcium solution containing 10 mmol/L HEPES, 135 mmol/L NaCl, 6 mmol/L KCl, 0.05 mmol/L CaCl2, 1.2 mmol/L MgCl2, 10 mmol/L Glucose, pH 7.4. The pieces of muscles were then transferred to low calcium solution containing 3 g/L papain, 2 g/L DTT, 2 g/L bovine serum albumin. Tissues were incubated at 36 °C in enzyme solution for 15 min and then suspended in enzyme-free low calcium solution. Tissue pieces were gently agitated to create a cell suspension. Dispersed cells were stored at 4 °C for later use. Experiments were performed at 20-22 °C within 10 h.
Relaxed single colon smooth muscle cells with smooth appearance and spindle shape observed under an inverted microscope (IX70, Olympus) were used. Myocytes were perfused with Ca2+-free cell bath solution in a self-made small volume chamber in which solution could be exchanged in 30 s. The composition of bath solution was the same as that of low calcium solution except for exclusion of calcium and inclusion of 0.2 mmol/L CdCl2 to minimize the contamination of calcium-dependent potassium currents.
Patch clamp micropipettes were pulled with a programmable puller (P-97, Sutter Instruments) and their tips were fire polished (CPM-2, ALA Co.). The pipette resistance was 3-5 MΩ. Currents were amplified with Axopatch 200B (Axon Instruments). Data were filtered at 1 kHz and analyzed with pClamp software (version 8.2). Nystatin-perforated whole cell patch clamp technique was used to record voltage-gated potassium currents. Nystatin was dissolved in DMSO at a concentration of 25 g/L, and then added to the internal pipette solution to yield a final nystatin concentration of 100 mg/L. The internal solution contained 10 mmol/L HEPES, 110 mmol/L glucoante (potassium salt), 30 mmol/L KCl, 10 mmol/L NaCl, 2 mmol/L MgCl2, pH 7.2. After giga seals were obtained, access resistance was monitored for 10 min to allow the drop of access resistance (average 17.7 ± 5.7 MΩ, n = 28) and then compensated at 70%. Macroscopic current values were normalized for cell capacitance as whole cell current densities (pA/pF). The average cell capacitance was 41.3±7.5 pF (n = 28 cells).
Papain, 4-aminopyridine (4-AP), nystatin, tetraethylammonium (TEA), sodium orthovanadate (VAN), genistein (GST) were purchased from Sigma. GST was prepared as a 50 mmol/L stock solution in DMSO and stored at -20 °C. DTT (BBI), HEPES, DMSO, bovine serum albumin were from Shanghai Sangon Biological Engineering Technology and Services Company.
Data were expressed as mean±SD. Differences in the data were evaluated by paired or independent t-test when appropriate. P<0.05 was considered statistically significant. Software Microcal Origin 5.0 was used for statistical analysis and graph plotting.
Outward potassium currents are mainly composed of voltage-gated potassium current and calcium-dependent potassium current. Under the conditions of our recordings (no added Ca2+ and 0.2 mmol/L CdCl2 in the bath solution), contamination of currents through calcium-dependent potassium currents was minimized.
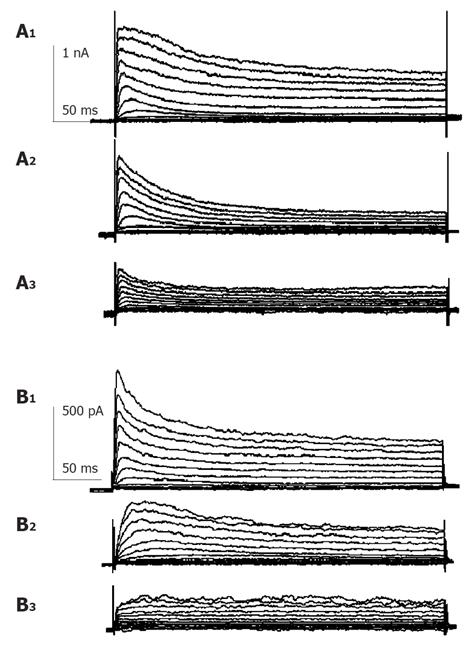
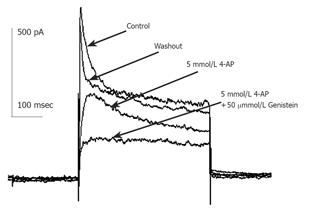
Currents were evoked using standard stimulus protocol, i.e., the membrane potential was stepped for 400 ms from a holding potential of -80 mV to test potentials between -80 and +50 mV in 10 mV increments. Depolarization to potentials positive to -40 mV activated non-linear, time-dependent outward currents which could be divided into transient outward potassium current (Ikto) and delayed rectifier potassium current (IKdr) as shown in Figure 1. Kto was sensitive to millimolar concentration of 4-AP and insensitive to TEA. Kdr was on the contrary. When 10 mmol/L TEA was externally applied, early peak current of transient outward current (peak current among the first 50 ms of test pulse, Ipeak) was only slightly decreased, but the quasi steady state current (average current from 350 to 400 ms after test pulse onset, ISS) was blocked -60% (Figure 1A). When 5 mmol/L 4-AP was externally applied, Ipeak was much reduced (Figure 1B) and the time to half-maximum current at test potential was significantly increased (data not shown), ISS was little affected. Detailed description of voltage-dependent potassium current of guinea pig proximal colon smooth muscle cells could be seen elsewhere[13], which agrees well with our study. According to the different sensitivity to TEA and 4-AP, we isolated these two kinds of current pharmacologically to study the effect of GST independently.
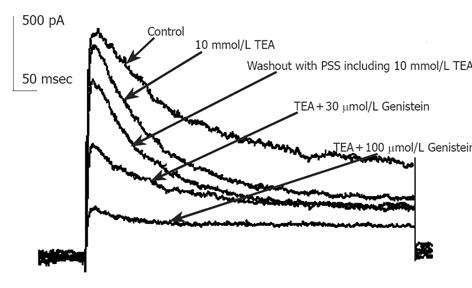
Currents recorded were mainly IKto when 10 mmol/L TEA was externally applied to bath solution. Repetitive single current traces were elicited for 400 ms from a holding potential of -80 mV to test potentials +50 mV in every 20s until stable currents were recorded. Ipeak of Kto decreased progressively and reached a stable state in about 1-2 min after the external perfusion of GST. GST (10-100 µmol/L) induced a reduction in Ipeak, with little effect on the steady state potassium current. The inhibitory effects of GST were reversible (Figure 2). Perfusion of calcium-free saline including TEA could restore the amplitude of currents to about 80% of control in 2 min (data not shown).
GST could not totally block the outward potassium currents even when 100 µmol/L GST was applied. Since no significant difference in the inhibitory effect was found between 50 (n = 10) and 100 (n = 5) µmol/L GST, a fraction of the outward potassium current was insensitive to GST.
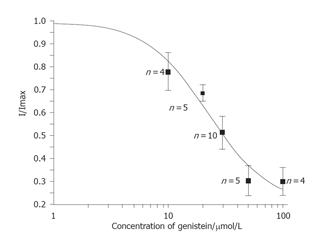
Early peak transient outward potassium current of Kto measured at +50 mV test pulse from a holding potential of -80 mV was used as an index of inhibition, which was plotted as a function of GST concentration (10-100 µmol/L). The data were fitted with IGST/Icontrol = 1/{1+(IC50/[D])n}, where [D] is the concentration of GST used, IC50 is the concentration at half maximal inhibition, and n is the Hill coefficient. Figure 3 shows that the effect of GST on Ipeak of Kto was concentration-dependent, with an IC50 of 22.0±6.9 µmol/L.
Currents were evoked using standard stimulus protocol. The membrane potential was stepped for 400 ms from a holding potential of -80 mV to test potentials between -80 and +50 mV in 10 mV increments. The interval between two pulses was set at 10 s to allow inactivated conductance to recover. Ipeak and ISS were measured at test pulses from -80 to +50 mV and converted into current densities, which were plotted as a function of test pulse.
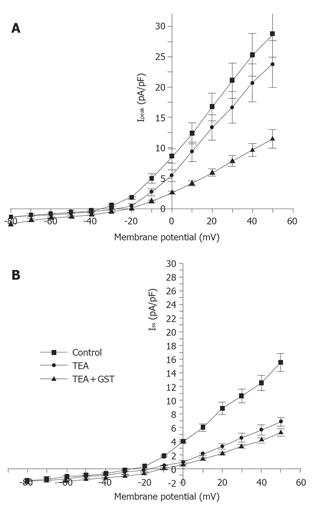
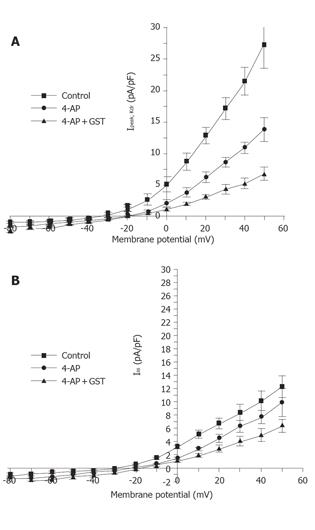
Figure 1 shows that in the presence of 10 mmol/L TEA, 30 µmol/L GST significantly blocked Ipeak (P<0.05, n = 6 cells) and only slightly reduced ISS. Figure 4 shows the averaged I-V curves of IKto inhibited by GST. The inhibition showed no voltage dependence.
Currents recorded in the presence of external 5 mmol/L 4-AP were mainly delayed rectifier potassium currents. Repetitive single current traces were elicited for 400 ms from a holding potential of -80 mV to test potentials +50 mV in every 20 s until stable currents were recorded. In the presence of 5 mmol/L 4-AP, 50 µmol/L GST reduced peak current and steady state current with a fractional inhibition of current f [f = (1-IGST/Idrug)×100%] of 52±8% (P<0.05, n = 4) and 33±2% (P<0.05, n = 4), respectively. The inhibitory effects of GST were reversible. Perfusion of calcium-free saline could restore the amplitude of currents in 2 min (data not shown). Details are shown in Figure 5.
The same experimental protocol as that of the study on the effect of GST on IKto was used. Figure 1B shows that in the presence of 5 mmol/L 4-AP, 50 µmol/L GST significantly blocked Ipeak as well as ISS (P<0.05, n = 4 cells). Figure 6 shows the averaged I-V curves of IKdr inhibited by GST. The inhibition showed no voltage dependence, and 50 µmol/L GST had a greater effect on IKto than on IKdr (P<0.05).
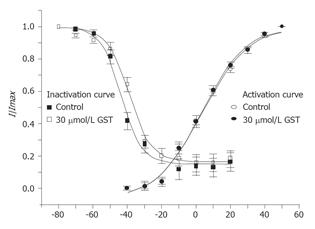
The activation curves of IKto were derived from I-V curves of IKto. GKto was calculated by dividing the initial peak current value by their driving force and plotted as a function of membrane potential. The activation curves were fitted with a Boltzmann function G/Gmax = 1/{1+exp[(V-Vh)]/k}, where V is membrane potential, Vh is the half-maximal activation voltage, and k is the slope constant (mV). GST had no significant effect on steady state activation kinetics of IKto. The value of half-maximal activation voltage Vh was 3.3±1.3 and 3.5±1.4 mV, respectively in the absence and presence of 30 µmol/LGST, the slope constant k was 12.9±1.0 and 13.3±1.1 mV, respectively (n = 6, Figure 7).
Classic double pulse protocol was used to determine the voltage dependence of inactivation of IKto. Currents were elicited by a depolarizing pulse of +50 mV with 1 s preconditioning pulses from -80 to 20 mV by increment of 10 mV. The interval between two sweeps was set at 20s. A plot of normalized peak current (I/Imax) as a function of preconditioning potential was fitted with a Boltzmann function: I/Imax = 1/{1+exp[(Vh-V)]/k} , where V is the membrane potential, Vh is the half-maximal inactivation voltage, and k is the slope constant (mV). GST had no significant effect on steady state inactivation kinetics of IKto. The values of half-maximal inactivation voltage Vh were -43.4±0.7 and -39.2±0.8 mV, respectively in the absence and presence of 30 µmol/L GST, the slope constant k was 5.8±0.6 and 6.5±0.7 mV, respectively (n = 6, Figure 7).
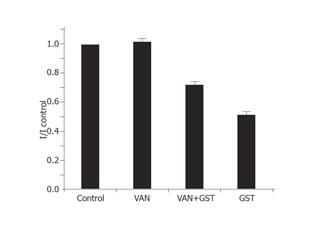
Inhibition of phosphatase-mediated tyrosine dephosphorylation would be expected to block current inhibition by GST, since dephosphorylation in the presence of GST would require ongoing phosphatase activity[14]. At commonly used bath concentrations of 0.1-10 mmol/L, inhibitor of tyrosine phosphatase orthovanadate could antagonize various GST-induced cellular responses such as blocking the inhibition of ICa,L by PTK inhibitors[15]. Solely applied VAN had minor stimulating but no significant effect on IKto (n = 7). In the presence of 1 mmol/L VAN, the fractional inhibition of current f of 30 µmol/LGST significantly decreased to 28±6% (Figure 1) compared with 49±6% of solely applied GST (n = 10). The rapid onset and offset responses to GST did not change (Figure 8).
At least four types of potassium channel have been identified in excitable gastrointestinal smooth muscle cells, such as voltage-dependent outward potassium channel, calcium-dependent potassium channel, ATP-sensitive potassium channel, and inward rectifier potassium channel. A given smooth muscle cell can express several families of potassium channels and several members of a single family of channels. This complexity is necessary for the control of smooth muscle function[11]. Kv channels are of particular importance in the regulation of colonic smooth muscle electrical activity because they provide outward currents over the voltage range in which these tissues operate[11]. Kv shapes the action potential (AP) by controlling its repolarization phase and determines the membrane potential and duration of the interspike interval. Delayed rectifier potassium channels keep single AP short and permit high-frequency trains of APs. Transient outward potassium channels help a cell fire at low frequency and promote broadening of APs during repetitive activity[16]. In murine colon, application of 4-AP to intact preparations can abolish the quiescent periods between slow waves and induce a slight depolarization[11]. IKto is fully inactivated during the upstroke depolarization[17]. In this study, GST could block transient outward potassium currents to depolarize membrane potential as well as delayed rectifier potassium currents to induce slight depolarization[11], thus modulating the contraction of smooth muscle.
Protein tyrosine kinase activity is a major signaling mechanism in regulating long-term processes such as cell growth, division, and metabolism[18]. PTK signaling is also important in regulating ion channel conductance. GST, a natural isoflavone which is abundant in soybean, is a specific inhibitor of tyrosine specific kinases by competing with ATP to form the nonproductive enzyme-substrate complexes[19]. In this study, GST concentration - dependently and reversibly blocked voltage-gated potassium currents. Though it was reported that GST has no specific effects such as direct interaction with potassium channels[14,20,21], orthovanadate could antagonize the blockage of GST on IKto, suggesting that although direct blockage of potassium channels by a mechanism unrelated to PTK inhibition could not be entirely excluded in this study, GST blocks the transient outward potassium channels partly via PTK pathway.
There are some differences in GST action on ion channel currents and contraction of different types of smooth muscle. GST can inhibit visceral smooth muscle contraction induced by vanadate[8], angiotensin II[9] and carbachol[10] as well as nifidipine-sensitive calcium currents in rabbit colon myocytes[22]. GST also can inhibit apamin-sensitive relaxation of the longitudinal muscle in rat distal colon induced by pituitary adenylate cyclase activating peptide[23] as well as potassium channels[4,5]. Besides species and tissue differences, it is possible that multiple receptor and nonreceptor PTKs are involved in the regulation of smooth muscle contraction. Further studies are required to identify the physiological role of PTKs in gastrointestinal motility.
To check whether GST could affect the biophysical kinetics of voltage-dependent potassium channels, we examined the steady state activation and inactivation kinetics in the absence and presence of 30 µmol/L GST. Peretz et al[4] found that GST affects potassium gating properties of Schwann cells such as a positive shift in voltage dependence of activation (by + 30 mV) and a decrease in steepness of activation gating. In our experiment, no significant effect of GST on gating properties of potassium channels was observed. Whether species or cell type difference contributes to the variation needs further investigation.
In conclusion, GST concentration dependently and reversibly inhibits transient outward potassium currents.
S- Editor Wang XL and Guo SY L- Editor Elsevier HK E- Editor Liu WF
| 1. | Davis MJ, Wu X, Nurkiewicz TR, Kawasaki J, Gui P, Hill MA, Wilson E. Regulation of ion channels by protein tyrosine phosphorylation. Am J Physiol Heart Circ Physiol. 2001;281:H1835-H1862. [PubMed] |
| 2. | Hopfield JF, Tank DW, Greengard P, Huganir RL. Functional modulation of the nicotinic acetylcholine receptor by tyrosine phosphorylation. Nature. 1988;336:677-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 204] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 3. | Wang YT, Salter MW. Regulation of NMDA receptors by tyrosine kinases and phosphatases. Nature. 1994;369:233-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 490] [Cited by in RCA: 544] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 4. | Peretz A, Sobko A, Attali B. Tyrosine kinases modulate K+ channel gating in mouse Schwann cells. J Physiol. 1999;519 Pt 2:373-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Holmes TC, Fadool DA, Levitan IB. Tyrosine phosphorylation of the Kv1.3 potassium channel. J Neurosci. 1996;16:1581-1590. [PubMed] |
| 6. | Ji ES, Yin JX, Ma HJ, He RR. Effect of genistein on L-type calcium current in guinea pig ventricular myocytes. Shengli Xuebao. 2004;56:466-470. [PubMed] |
| 7. | Strauss O, Mergler S, Wiederholt M. Regulation of L-type calcium channels by protein tyrosine kinase and protein kinase C in cultured rat and human retinal pigment epithelial cells. FASEB J. 1997;11:859-867. [PubMed] |
| 8. | Alcón S, Camello PJ, García LJ, Pozo MJ. Activation of tyrosine kinase pathway by vanadate in gallbladder smooth muscle. Biochem Pharmacol. 2000;59:1077-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Yang SG, Saifeddine M, Laniyonu A, Hollenberg MD. Distinct signal transduction pathways for angiotensin-II in guinea pig gastric smooth muscle: differential blockade by indomethacin and tyrosine kinase inhibitors. J Pharmacol Exp Ther. 1993;264:958-966. [PubMed] |
| 10. | Di Salvo J, Steusloff A, Semenchuk L, Satoh S, Kolquist K, Pfitzer G. Tyrosine kinase inhibitors suppress agonist-induced contraction in smooth muscle. Biochem Biophys Res Commun. 1993;190:968-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 133] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 11. | Koh SD, Ward SM, Dick GM, Epperson A, Bonner HP, Sanders KM, Horowitz B, Kenyon JL. Contribution of delayed rectifier potassium currents to the electrical activity of murine colonic smooth muscle. J Physiol. 1999;515:475-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Komori S, Bolton TB. Role of G-proteins in muscarinic receptor inward and outward currents in rabbit jejunal smooth muscle. J Physiol. 1990;427:395-419. [PubMed] |
| 13. | Vogalis F, Lang RJ, Bywater RA, Taylor GS. Voltage-gated ionic currents in smooth muscle cells of guinea pig proximal colon. Am J Physiol. 1993;264:C527-C536. [PubMed] |
| 14. | Smirnov SV, Aaronson PI. Inhibition of vascular smooth muscle cell K+ currents by tyrosine kinase inhibitors genistein and ST 638. Circ Res. 1995;76:310-316. [PubMed] |
| 15. | Ogura T, Shuba LM, McDonald TF. L-type Ca2+ current in guinea pig ventricular myocytes treated with modulators of tyrosine phosphorylation. Am J Physiol. 1999;276:H1724-H1733. [PubMed] |
| 16. | Oliver D, Lien CC, Soom M, Baukrowitz T, Jonas P, Fakler B. Functional conversion between A-type and delayed rectifier K+ channels by membrane lipids. Science. 2004;304:265-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 258] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 17. | Amberg GC, Baker SA, Koh SD, Hatton WJ, Murray KJ, Horowitz B, Sanders KM. Characterization of the A-type potassium current in murine gastric antrum. J Physiol. 2002;544:417-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Fantl WJ, Johnson DE, Williams LT. Signalling by receptor tyrosine kinases. Annu Rev Biochem. 1993;62:453-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 728] [Cited by in RCA: 699] [Article Influence: 21.8] [Reference Citation Analysis (1)] |
| 19. | Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe S, Itoh N, Shibuya M, Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987;262:5592-5595. [PubMed] |
| 20. | Paillart C, Carlier E, Guedin D, Dargent B, Couraud F. Direct block of voltage-sensitive sodium channels by genistein, a tyrosine kinase inhibitor. J Pharmacol Exp Ther. 1997;280:521-526. [PubMed] |
| 21. | Belevych AE, Warrier S, Harvey RD. Genistein inhibits cardiac L-type Ca(2+) channel activity by a tyrosine kinase-independent mechanism. Mol Pharmacol. 2002;62:554-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Hatakeyama N, Mukhopadhyay D, Goyal RK, Akbarali HI. Tyrosine kinase-dependent modulation of calcium entry in rabbit colonic muscularis mucosae. Am J Physiol. 1996;270:C1780-C1789. [PubMed] |
| 23. | Takeuchi T, Kishi M, Hirayama N, Yamaji M, Ishii T, Nishio H, Hata F, Takewaki T. Tyrosine kinase involvement in apamin-sensitive inhibitory responses of rat distal colon. J Physiol. 1999;514:177-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |