Published online Jan 21, 2006. doi: 10.3748/wjg.v12.i3.403
Revised: June 30, 2005
Accepted: July 29, 2005
Published online: January 21, 2006
AIM: To investigate the role of Kupffer cells (KCs) in acute hemorrhagic necrotizing pancreatitis-associated lung injury (AHNP-LI).
METHODS: Forty-two rats were allocated to four groups [sham operation, AHNP model, gadolinium chloride (GdCl3) pretreatment, GdCl3 control]. In GdCl3 pretreatment group, GdCl3 was administered by caudal vein injection 24 h before the AHNP model induction. Blood from the iliac artery, alveolar macrophages and tissues from the pancreas and lung, were collected in six animals per group 3 and 6 h after acute pancreatitis induction. TNF-α, IL-1 of serum, myeloperoxidase (MPO) of lung tissue, NF-κB activation of alveolar macrophages were detected. Serum AST and ALT in sham operation group and GdCl3 control group were tested. In addition, histopathological changes of the pancreas and lung were observed under light microscope.
RESULTS: MPO of lung tissue and TNF-α, IL-1 levels of serum were all reduced significantly in GdCl3 pretreatment group compared to those in AHNP group (P <0.01). NF-κB activation of alveolar macrophages was also attenuated significantly in GdCl3 pretreatment group compared to that in AHNP group (P <0.01). The pathological injury of the lung was ameliorated obviously in GdCl3 pretreatment group compared to that in AHNP group. Nevertheless, the serum amylase level did not reduce and injury of the pancreas was not prevented in GdCl3 pretreatment group.
CONCLUSION: Pulmonary injury induced by AHNP is mediated by KC activation and AHNP-LI can be significantly ameliorated by pretreatment with GdCl3 and KCs play a vital role in AHNP-LI.
- Citation: Liu HB, Cui NQ, Li DH, Chen C. Role of Kupffer cells in acute hemorrhagic necrotizing pancreatitis-associated lung injury of rats. World J Gastroenterol 2006; 12(3): 403-407
- URL: https://www.wjgnet.com/1007-9327/full/v12/i3/403.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i3.403
Acute lung injury is a severe complication of acute hemorrhagic necrotizing pancreatitis (AHNP)[1-3]. However, its pathogenic mechanism is not well understood and its treatment remains supportive. Recent researches suggest that inflammatory cytokines derived from the liver, especially hepatic cytokine released from Kupffer cells (KCs) may cause distant organ failure and death in severe pancreatitis and that KCs are an important source of inflammatory cytokines and may be the main factor causing lung damage in AHNP[4,5]. Here we studied the KC contribution to lung injury associated with AHNP. The present study was to investigate the role of KCs in acute hemorrhagic necrotizing pancreatitis-associated lung injury.
Male Wistar rats were provided by Experimental Animal Center of Capital Medical University (Beijing). Sodium taurocholate and gadolinium chloride (GdCl3) were purchased from Sigma-Aldrich (St. Louis, MO, USA). TNF-α, IL-1 ELISA kits were purchased from TPI Ltd. (USA). TransAMTM NF-κB P65 chemi ELISA kit was provided by Active Motif (USA). Central LB 960 microplate luminometer was from Berthold Ltd.
Experimental AHNP was induced as previously described[6]. Briefly, a small median laparotomy was performed, then the pancreas was exteriorized and the hepatic duct was closed at the liver hilum with a soft microvascular clamp to prevent reflux of the infused material into the liver. The biliopancreatic duct was cannulated through the duodenum and 5% sodium taurocholate (1 mL/kg body weight, 0.1 mL/min) was retrogradely injected into the biliopancreatic duct. The clamp was removed 5 min after the injection. The abdominal wound was closed, and the animals were sent back to their cages with free access to water and food after surgery.
Forty-two male Wistar rats (weighing 250-280 g) were randomly divided into four groups: sham operation, AHNP model, GdCl3 pretreatment and GdCl3 control. In the GdCl3 pretreatment group, GdCl3 solution (4%, 10 mg/kg) was administrated by caudal vein injection 24 h before the AHNP model was established. Blood from the iliac artery, alveolar macrophages, and tissues from the pancreas and lung, were collected in six animals per group 3 and 6 h after acute pancreatitis induction. Serum levels of TNF-α and IL-1 were determined by enzyme-linked immunosorbent assay. Myeloperoxidase (MPO) level in the lung and NF-κB activation of the alveolar macrophages were detected. Serum AST and ALT in sham operation group and GdCl3 control group were tested by biochemical method. In addition, histopathological changes of the pancreas and lung were observed under light microscope.
Bronchoalveolar lavage (BAL) was performed five times in the left lung, using 5 mL of sterile normal saline per lavage given through a tracheal cannula[7]. The whole BAL fluid (BALF) was centrifuged at 280 r/min for 10 min at 4 °C. Cell pellets were resuspended (1×105 cells/mL) in RPMI 1640 medium. Cell suspension was then placed in plastic petri dishes (Nunc, Denmark) and incubated at 37 °C for 1 h in a CO2 incubator (50 mL/L CO2+95% air). Non-adherent cells were removed from adherent macrophages by washing with RPMI 1640 medium. Purified alveolar macrophages were recovered by gently rubbing the dishes with a rubber policeman. Nuclear protein was extracted from purified alveolar macrophages as previously described[8]. Protein content was determined by Bradford method, stored at -70 °C for subsequent examination of NF-κB activity.
Serum amylase activity was measured by iodoamylum method and expressed as U/L.
Serum TNF-α and IL-1 were measured by ELISA method according to the instructions of the kits.
Lung tissue MPO activity was detected according to the instructions of commercial kit.
Serum levels of AST and ALT were determined using Toshiba VF-A5/A5P Bio-Chemical analyzer.
NF-κB activation of alveolar macrophages was determined using TransAMTM NF-κB P65 chemi ELISA kit by Central LB 960 microplate luminometer and expressed as relative light units (RLU).
Pancreas and lung tissues were collected and evaluated by a pathologist blinded to the experimental assignment of the animals. After embedded with paraffin, the tissue was stained with hematoxylin-eosin (H&E). Pulmonary lesion was scored following Lei’s criteria[9].
All data were expressed as mean±SD. Comparisons among multiple experimental groups and between each time point were made using ANOVA. P<0.05 was considered statistically significant.
Table 1 illustrates the levels of serum amylase in all the three groups. Three and six hours after AHNP induction, serum amylase increased significantly in AHNP group compared to that in sham operation group. But there was no significant difference between GdCl3 pretreatment group and AHNP group 3 and 6 h after AHNP induction.
Table 2 shows the influence of GdCl3 on hepatic functions of experimental animals. No changes were found in GdCl3-treated animals and the values of ALT and AST in GdCl3 control group were similar to those in sham group measured at all time points.
| Group | n | Serum AST | Serum ALT |
| Sham 3 h | 6 | 74.2 ± 13.6 | 31.6 ± 9.3 |
| Sham 6 h | 6 | 71.3 ± 15.0 | 32.7 ± 11.3 |
| GdCl3 control | 6 | 82.5 ± 14.6 | 28.9 ± 8.7 |
Serum TNF-α and IL-1 in AHNP group were significantly increased compared to those measured in sham group at all time points (P<0.01). In GdCl3 pretreatment group, 3 and 6 h after AHNP induction, serum TNF-α and IL-1 were significantly decreased (P<0.01, Table 3).
Table 4 shows the MPO activity of lung injury in all the groups. The MPO levels in AHNP group were significantly higher than those in sham operation group (P < 0.01). In GdCl3 pretreatment group, 3 and 6 h after AHNP induction, the lung MPO levels were significantly decreased (P < 0.01).
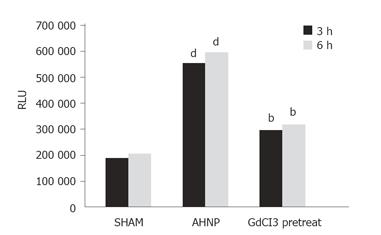
Figure 1 shows that the NF-κB activity of alveolar macrophages in AHNP group was significantly higher than that in sham operation group (P < 0.01) and was significantly decreased in GdCl3 pretreatment group (P < 0.01).
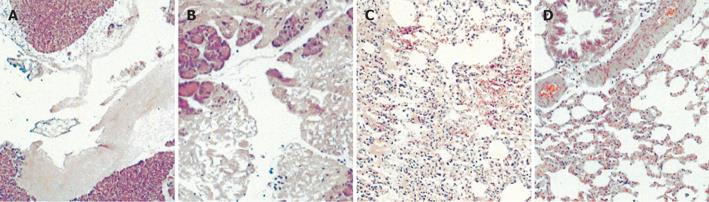
Histopathological study of the pancreas, 3 and 6 h after AHNP induction (Figure 2A) revealed extensive necrosis of pancreatic tissue, intense edema, and inflammatory infiltrate. The necrosis of pancreatic tissue and edema in GdCl3 pretreatment group were similar to those in AHNP group (Figure 2B). The sham operation group was normal.
Diffuse alveolar blood stasis, intense alveolar septum swelling and heavy infiltration of inflammatory cells mostly neutrophils were found in the lung tissue of AHNP group (Figure 2C). The mean histopathologic scores of AHNP group were significantly higher than those in sham operation group (P < 0.01, Table 5). In GdCl3 pretreatment group, the major histopathological findings were mild edema of the alveolar walls and mild alveolar blood stasis with slight infiltration of neutrophils (Figure 2D). The mean histopathologic scores of GdCl3 pretreatment group decreased significantly compared to those of AHNP group (P < 0.05).
Acute hemorrhagic necrotizing pancreatitis (AHNP) is a potentially fatal disease with a morbidity and mortality rate of approximately 30%. Acute lung injury (ALI) is a common complication of AHNP, but the events that link AHNP and pulmonary damage are not fully understood. Many factors, such as oxygen free radicals, platelet activating factor, phospholipase A2 (PLA2), cyclooxygenase-2 (COX-2), cytokines and arachidonic acid metabolites are related to AHNP and ALI[10-13]. Pancreatic proteolytic enzymes or activated PLA2 released into the circulatory system determines the development of lung injury[14]. Furthermore, other mediators in lung tissue such as platelet activating factor, arachidonic acid metabolites can stimulate inflammatory cell activation[15,16]. Also, interaction of polymorphonuclear granulocytes, endothelium, and endothelium-derived mediators seems to be important to amplify lung damage[17]. Recently, Cheng et al[18,19] noted that activation of alveolar macrophages may play an important role in lung injury associated with AHNP, and that TNF-α and nitric oxide (NO) secreted by alveolar macrophages are increased significantly in rats with AHNP. Bhatia et al[20] reported that inhibition of the production of hydrogen sulfide (H2S) can significantly reduce the severity of cerulein-induced pancreatitis and associated-lung injury, suggesting an important proinflammatory role in regulating the severity of pancreatitis and associated-lung injury.
In recent years, some researchers found that the liver, especially KCs, might play a vital role in ALI caused by other different factors. Okutan et al[21] reported that KCs blocked by GdCl3 can attenuate lung damage caused by aortic ischemia reperfusion and malondialdehyde (MDA) level, an indicator of free radical generation and MPO activity, an indirect evidence of neutrophil infiltration in lung injury are decreased significantly[21]. Although there is no evidence that GdCl3 can suppress the function of neutrophils, it was reported that GdCl3 can suppress the accumulation of neutrophils and alveolar macrophages[22]. Feng et al[23] investigated the role of KCs in the pathogenesis of ALI during acute obstructive cholangitis (AOC) and found that the phagocytic function of KCs is damaged in ALI induced by AOC.
KCs, the resident macrophages in the liver, are the major component of mononuclear phagocytic system (MPS). These macrophages make up 90% of the MPS and have abundant cytoplasma where abundant ribosome and phagosomes are located. These typical structures are associated with their functions. It was reported that KCs are responsible for the increased levels of TNF, IL-1, IL-6 in trauma, hemorrhagic shock and resuscitation. Decreasing the number or functional ability of KCs can lead to decreased levels of inflammatory cytokines as seen in the models of liver resection and sepsis[24,25].
Also, KCs are regarded as the predominant source of inflammatory cytokines in AHNP at present[26,27]. Closa et al[4,5] performed an end-to-side portacaval shunt before AHNP induction in rats and found that portacaval shunting appears to exert a profound effect on ameliorating the inflammatory infiltrate. It is suggested that almost all the pancreatic enzymes and mediators released from the pancreas into the plasma during AHNP pass through the liver before their dilution in the systemic circulation, indicating that this step is a determinant in the development of the lung injury response. These observations point to the key role of liver as a triggering mechanism for inflammatory processes in the lung as a consequence of AHNP. Moreover, activation of hepatic inflammatory cells, especially KCs, plays a key role in the development of lung injury[4,5].
To improve our understanding of the role of KCs in AHNP-ALI, GdCl3 was given 24 h prior to AHNP model induction to eliminate KCs in the present study. Moreover, the dose of GdCl3 used in our study was 10 mg/Kg because administration of GdCl3 at a dose 10 mg/kg can block the phagocytic activity of KCs completely[28]. In GdCl3 pretreatment group, the levels of MPO in lung tissue and the levels of TNF-α, IL-1 in serum all decreased significantly compared to those in AHNP group. NF-κB activation of alveolar macrophages was also attenuated significantly in GdCl3 pretreatment group compared to that in AHNP group. All these data suggest that GdCl3 can ameliorate AHNP-ALI significantly and these results are in accordance with those reported by Folch et al[29]. It was reported that lung injury associated with acute necrotic pancreatitis (ANP) is ameliorated by GdCl3 through inducing apoptosis of alveolar macrophages of ANP[30].
In our study, the serum amylase level did not decrease and the injury of pancreas was not prevented in GdCl3 pretreatment group, suggesting that GdCl3 cannot prevent lung injury by ameliorating pancreatic injury.
Using GdCl3 as a tool to investigate the role of KCs is more reasonable than portacaval shunting operation which appears to exert a profound effect on animal’s systemic circulation. Serum AST and ALT estimation suggests that GdCl3 has no harmful effects on hepatic functions.
In conclusion, KCs play a vital role in AHNP and ALI.
The authors thank Xiao-Ping Xue, Xiu-Zhu Yang, Qian Wang, and Yan-Ping Zhang, for their technical assistance.
S- Editor Wang XL and Guo SY L- Editor Elsevier HK E- Editor Liu WF
| 1. | 1 Zhao X, Andersson R, Wang X, Dib M, Wang X. Acute pancreatitis-associated lung injury: pathophysiological mechanisms and potential future therapies. Scand J Gastroenterol. 2002;37:1351-1358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 2. | Liu YG, Pan JJ, Niu LC, Yan QM, Song B, Li Y, Zhang WF. 18 cases report of severe acute pancreatitis-associated lung injury. Zhongguo Putong Waike Zazhi. 2002;11:182-184. |
| 3. | Wang Y, Tian YX, Tan XD. The therapeutic methods of severe acute pancreatitis (94 cases report). Zhongguo Putong Waike Zazhi. 2001;10:302-304. |
| 4. | Closa D, Sabater L, Fernández-Cruz L, Prats N, Gelpí E, Roselló-Catafau J. Activation of alveolar macrophages in lung injury associated with experimental acute pancreatitis is mediated by the liver. Ann Surg. 1999;229:230-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 88] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 5. | Closa D, Bardají M, Hotter G, Prats N, Gelpí E, Fernández-Cruz L, Roselló-Catafau J. Hepatic involvement in pancreatitis-induced lung damage. Am J Physiol. 1996;270:G6-13. [PubMed] |
| 6. | Aho HJ, Koskensalo SM, Nevalainen TJ. Experimental pancreatitis in the rat. Sodium taurocholate-induced acute haemorrhagic pancreatitis. Scand J Gastroenterol. 1980;15:411-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 257] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 7. | Satoh A, Shimosegawa T, Fujita M, Kimura K, Masamune A, Koizumi M, Toyota T. Inhibition of nuclear factor-kappaB activation improves the survival of rats with taurocholate pancreatitis. Gut. 1999;44:253-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 109] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 8. | Schreiber E, Matthias P, Müller MM, Schaffner W. Rapid detection of octamer binding proteins with 'mini-extracts', prepared from a small number of cells. Nucleic Acids Res. 1989;17:6419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3329] [Cited by in RCA: 3628] [Article Influence: 100.8] [Reference Citation Analysis (0)] |
| 9. | Lei WZ, Wei JJ, Shen WL, Jin LR. The relationship between MODS of experimental necrotizing pancreatitis and endotoxinmia. Zhongguo Shiyan Waike Zazhi. 1995;12:131-133. |
| 10. | Hardman J, Shields C, Schofield D, McMahon R, Redmond HP, Siriwardena AK. Intravenous antioxidant modulation of end-organ damage in L-arginine-induced experimental acute pancreatitis. Pancreatology. 2005;5:380-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Takala A, Jousela I, Takkunen O, Kautiainen H, Jansson SE, Orpana A, Karonen SL, Repo H. A prospective study of inflammation markers in patients at risk of indirect acute lung injury. Shock. 2002;17:252-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 66] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 12. | Ethridge RT, Chung DH, Slogoff M, Ehlers RA, Hellmich MR, Rajaraman S, Saito H, Uchida T, Evers BM. Cyclooxygenase-2 gene disruption attenuates the severity of acute pancreatitis and pancreatitis-associated lung injury. Gastroenterology. 2002;123:1311-1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 93] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 13. | Song AM, Bhagat L, Singh VP, Van Acker GG, Steer ML, Saluja AK. Inhibition of cyclooxygenase-2 ameliorates the severity of pancreatitis and associated lung injury. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1166-G1174. [PubMed] |
| 14. | Edelson JD, Vadas P, Villar J, Mullen JB, Pruzanski W. Acute lung injury induced by phospholipase A2. Structural and functional changes. Am Rev Respir Dis. 1991;143:1102-1109. [PubMed] |
| 15. | St John RC, Dorinsky PM. Immunologic therapy for ARDS, septic shock, and multiple-organ failure. Chest. 1993;103:932-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 70] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Zhou W, Chao W, Levine BA, Olson MS. Role of platelet-activating factor in hepatic responses after bile duct ligation in rats. Am J Physiol. 1992;263:G587-G592. [PubMed] |
| 17. | Willemer S, Feddersen CO, Karges W, Adler G. Lung injury in acute experimental pancreatitis in rats. I. Morphological studies. Int J Pancreatol. 1991;8:305-321. [PubMed] |
| 18. | Cheng S, He S, Zhang J. [The role of alveolar macrophage activation in rats with lung injury associated with acute necrotizing pancreatitis]. Zhonghua Waike Zazhi. 2002;40:609-612. [PubMed] |
| 19. | Cheng S, Zhao J, He SG, Song MM, Li ZH, Zhang YW. [The role of nitric oxide in lung injury associated with acute necrotizing pancreatitis]. Zhonghua Wai Ke Za Zhi. 2003;41:336-339. [PubMed] |
| 20. | Bhatia M, Wong FL, Fu D, Lau HY, Moochhala SM, Moore PK. Role of hydrogen sulfide in acute pancreatitis and associated lung injury. FASEB J. 2005;19:623-625. [PubMed] |
| 21. | Okutan H, Savas C, Ozguner IF, Yonden Z, Eren VC, Delibas N. Lung injury after aortic occlusion-reperfusion in rats: the role of gadolinium chloride. Tohoku J Exp Med. 2004;203:267-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Sato K, Kadiiska MB, Ghio AJ, Corbett J, Fann YC, Holland SM, Thurman RG, Mason RP. In vivo lipid-derived free radical formation by NADPH oxidase in acute lung injury induced by lipopolysaccharide: a model for ARDS. FASEB J. 2002;16:1713-1720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 143] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 23. | Feng HY, Shi YJ, Wu CX, Li SW, Liu CA, Gong JP. Mononuclear macrophages in pathogenesis of acute lung injury during acute obstructive cholangitis. Hepatobiliary Pancreat Dis Int. 2002;1:587-591. [PubMed] |
| 24. | Savas C, Ozogul C, Karaöz E, Delibas N, Ozgüner F. Splenectomy reduces remote organ damage after intestinal ischaemia-reperfusion injury. Acta Chir Belg. 2003;103:315-320. [PubMed] |
| 25. | Towfigh S, Heisler T, Rigberg DA, Hines OJ, Chu J, McFadden DW, Chandler C. Intestinal ischemia and the gut-liver axis: an in vitro model. J Surg Res. 2000;88:160-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Gloor B, Blinman TA, Rigberg DA, Todd KE, Lane JS, Hines OJ, Reber HA. Kupffer cell blockade reduces hepatic and systemic cytokine levels and lung injury in hemorrhagic pancreatitis in rats. Pancreas. 2000;21:414-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 81] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 27. | Jankov RP, Luo X, Belcastro R, Copland I, Frndova H, Lye SJ, Hoidal JR, Post M, Tanswell AK. Gadolinium chloride inhibits pulmonary macrophage influx and prevents O(2)-induced pulmonary hypertension in the neonatal rat. Pediatr Res. 2001;50:172-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 56] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 28. | Tullis MJ, Brown S, Gewertz BL. Hepatic influence on pulmonary neutrophil sequestration following intestinal ischemia-reperfusion. J Surg Res. 1996;66:143-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Folch E, Prats N, Hotter G, López S, Gelpi E, Roselló-Catafau J, Closa D. P-selectin expression and Kupffer cell activation in rat acute pancreatitis. Dig Dis Sci. 2000;45:1535-1544. [PubMed] |
| 30. | Cheng S, Song MM, Li ZH, He SG. [The protective role of gadolinium chloride in lung injury associated with acute necrotizing pancreatitis]. Zhonghua Waike Zazhi. 2004;42:936-939. [PubMed] |