Published online Jul 28, 2006. doi: 10.3748/wjg.v12.i28.4498
Revised: March 12, 2006
Accepted: April 21, 2006
Published online: July 28, 2006
AIM: To investigate in vivo, whether CCK2 receptors (CCK2R) regulate proteins known to play a crucial role in cell proliferation and cancer development and analyse in vitro the molecular mechanisms that lead to Src activation; in particular, to identify the domains within the CCK2R sequence that are implicated in this activation.
METHODS: The expression and activation of Src and ERK were studied in vivo using immuno-fluorescence and western-blot techniques. We used pancreatic tissues derived from wild type or Elas-CCK2 mice that expressed the CCK2R in pancreatic acini, displayed an increased pancreatic growth and developed preneoplastic lesions. The pancreatic tumor cell line AR4-2J expressing the endogenous CCK2R or COS-7 cells transiently transfected with wild type or mutant CCK2R were used as in vitro models to study the mechanism of Src activation. Src activation was measured by in vitro kinase assays, ERK activation by western blot using anti-phospho-ERK antibodies and the involvement of Src in gastrin-induced cell proliferation by MTT test.
RESULTS: We showed in vivo that the targeted CCK2R expression in the pancreas of Elas-CCK2 mice, led to the activation of Src and the ERK pathway. Src was activated upstream of the ERK pathway by the CCK2R in pancreatic tumoral cells and contributed to the proliferative effects mediated by this receptor. In vitro results demonstrated that activation of the Src/ERK pathway by the CCK2R required the NPXXY motif, located within the CCK2R sequence at the end of the 7th transmembrane domain, and suggested the putative role of Gq in this mechanism.
CONCLUSION: Deregulation of the Src/ERK pathway by the CCK2R might represent an early step that contributes to cell proliferation, formation of preneoplastic lesions and pancreatic tumor development.
- Citation: Ferrand A, Vatinel S, Kowalski-Chauvel A, Bertrand C, Escrieut C, Fourmy D, Dufresne M, Seva C. Mechanism for Src activation by the CCK2 receptor: Patho-physiological functions of this receptor in pancreas. World J Gastroenterol 2006; 12(28): 4498-4503
- URL: https://www.wjgnet.com/1007-9327/full/v12/i28/4498.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i28.4498
The CCK2 receptor (CCK2R or CCKBR) is a G protein-coupled receptor, mainly coupled to Gq proteins[1]. Initially implicated in gastrin-mediated acid secretion CCK2R is now recognized to mediate mitogenic and anti-apoptotic effects on gastrointestinal and pancreatic cells[2]. In the transgenic mice, Elas-CCK2, CCK2R expression has been targeted in pancreatic acinar cells using transcriptional elements of the elastase-1 promoter[3]. Using this model, we have recently reported an increase in pancreatic growth as well as an acinar to ductal transdifferentiation, postulated to be a preneoplastic step in pancreatic carcinogenesis that precedes the development of tumours[3]. Similar observations have been reported in two other transgenic models overexpressing TGF- αor a Kras mutant in exocrine pancreas[4,5].
Src family kinases are non receptor protein tyrosine kinases that mediate a wide variety of biological effects including cell survival, adhesion and proliferation. They are activated by many growth factor receptors. In addition, p60-Src is well established as an oncogene, and overexpression of Src tyrosine kinase has been described in human pancreatic adenocarcinomas[6]. Recently, Src inhibition by AZM475271 demonstrated significant antitumorigenic and anti-metastatic activity in an orthotopic nude mouse model for human pancreatic cancer[7]. However to our knowledge, there is currently little or no information regarding the deregulation of Src kinases in early stages of pancreatic carcinogenesis.
It is well established that numerous G protein-coupled receptors activate Src family kinases. However, very few publications have described the molecular mechanisms involved in Src activation by this receptor family. Molecular mechanisms of Src activation by CCK2R as well as the role of this kinase and related signalling pathways in the pathophysiological functions of CCK2R in vivo remain largely unknown. This study had two main aims: First, to investigate in vivo, using the Elas-CCK2 mouse model, whether CCK2R is involved in the regulation of proteins known to play a crucial role in cell proliferation and cancer development; Second, to analyse in vitro the molecular mechanisms that lead to Src activation and in particular, to identify the domains within the CCK2R sequence implicated in this activation.
Homozygous Elas-CCK2 mice used in this study have been previously described[3]. At least 3 homozygous Elas-CCK2 mice in a B6SJLF1 background and 3 corresponding control littermate mice were used (six months old). Mice were reared in routine animal facility of the IFR31 and maintained on a 12:12 h light-dark cycle. All the experiments were performed during the daytime. All procedures were approved by the IFR31 animal facility care committee.
GAPDH was provided by Chemicon; phospho-tyr418-Src (IF and WB) by Biosource; ERK, Src (IF and WB) by Santa Cruz Biotechnology; phospho-ERK (IF and WB) by Cell Signaling; SRC (IP) by Oncogene Research Product; PP2, GP2A by Calbiochem.
Mice were killed by decapitation and the pancreas was excised, fixed in Bouin’s solution and embedded in paraffin using standard techniques. Immunofluorescence staining was performed as previously described[8]. The detection was done using secondary antibodies coupled to Alexa Fluor 488. Slides were analyzed on a Nikon E400 microscope with a Sony DXC 950 camera and Visiolab 2000 software. For semi-quantitative comparisons, identical volumes of antibody were used for all samples and identical exposure times taken.
Western-blot analyses were performed on dispersed acini from mouse pancreas prepared as previously described[9], and on cell lysates or immunoprecipitates from AR4-2J or COS7 cells stimulated or not with gastrin. Fractions, containing identical levels of proteins, were separated by SDS-PAGE and analyzed by western-blot with the indicated antibodies as described previously[9].
AR4-2J cells and COS-7 cells were grown in DMEM supplemented with 10% and 5% fetal calf serum (FCS), respectively, at 37°C in a 95% air, 5 mL/L CO2 atmosphere. For proliferation assays, an optimal number of AR4-2J cells (4 × 104 cells) were plated in 35-mm dishes, serum-starved for 24 h, then treated for 48 h with gastrin (10 nmol/L). When indicated, cells were incubated with PP2 (10 μmol/L). Cells were counted by using a Coulter electronic counter.
After gastrin stimulation, cells were lysed and Src immunoprecipitated with specific antibodies. Kinase assays were performed and analyzed as previously described[10]. Proteins were separated by SDS-PAGE and the gel autoradiographed.
Mutant CCK2R, N386A-CCK2R was previously described[9]. Plasmids coding for wild type or mutant CCK2R (6 μg) were transiently transfected into COS-7 cells using the DEAE/dextran method as described previously[1].
Data were expressed as mean ±SE and Student’s t test was performed using “GraphPad Prism”. P < 0.05 was taken as significant.
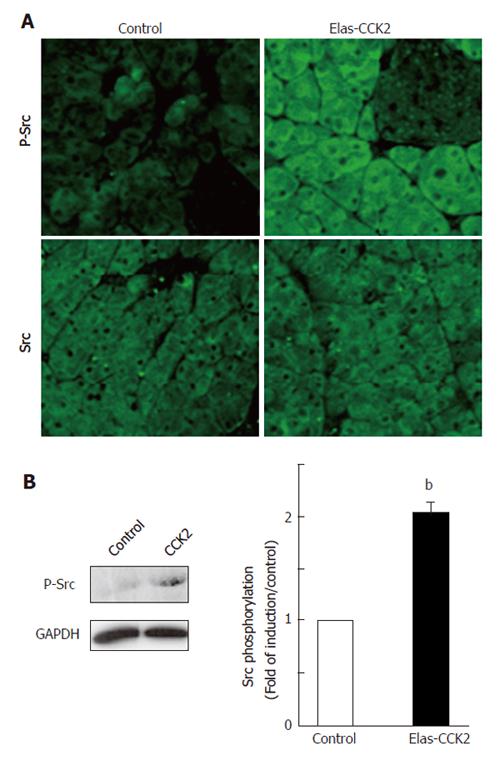
Src activation was analyzed by immunohistochemical methods on pancreatic sections from control and Elas-CCK2 mice of 6 month old using antibodies specific for total Src or detecting the activated form of the protein phosphorylated on tyrosine 418 (P-Src). Acinar tissues derived from Elas-CCK2 mice demonstrated higher levels of Src activation as compared to control mice (Figure 1A, upper panels). In contrast, total Src protein expression was unchanged in the two mouse models (Figure 1A, lower panels).
To confirm and quantify Src activation, western-blot analyses were performed on lysates of acinar cells isolated from pancreas of control and Elas-CCK2 mice. Src activation in Elas-CCK2 mice was significantly elevated compared to controls (Figure 1B). Thus, the expression of the CCK2R in mouse pancreatic acini induced Src activation.
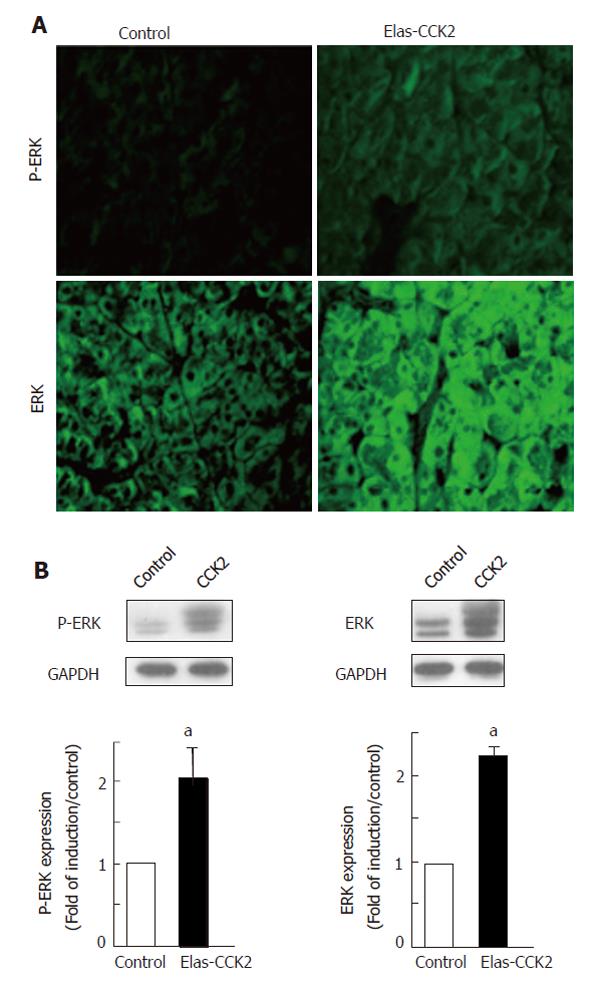
Activation of the ERK pathways by gastrin has previously been described in vitro[10,11]. However, there is currently no in vivo information about the status of the ERK pathways in gastrin signaling. Immunofluorescence analysis was performed using antibodies specific for dually phosphorylated active ERK (P-ERK). Results demonstrated an increased immunoreactivity in acinar tissue of transgenic mice as compared to control mice (Figures 2A, upper panel). In addition, pancreatic acinar tissues of Elas-CCK2 mice also showed a higher level of total ERK expression as compared to control mice (Figure 2A, lower panel). These results were confirmed by western-blots performed on lysates of acinar cells isolated from pancreas of control and Elas-CCK2 mice (Figure 2B). Overall, these results were consistent with an upregulation of the ERK pathway in the pancreas of mice expressing the CCK2R.
The pancreatic tumour cell line AR4-2J, that exhibits an acinar phenotype, was previously shown to express endogenous CCK2R[12]. We used this model to analyse the role of Src in the pathophysiological functions of the CCK2R and the molecular mechanisms potentially involved in Src activation by this G protein-coupled receptor.
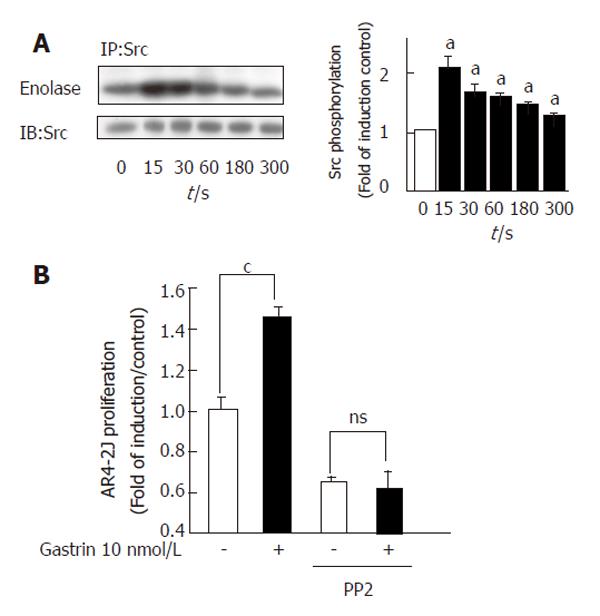
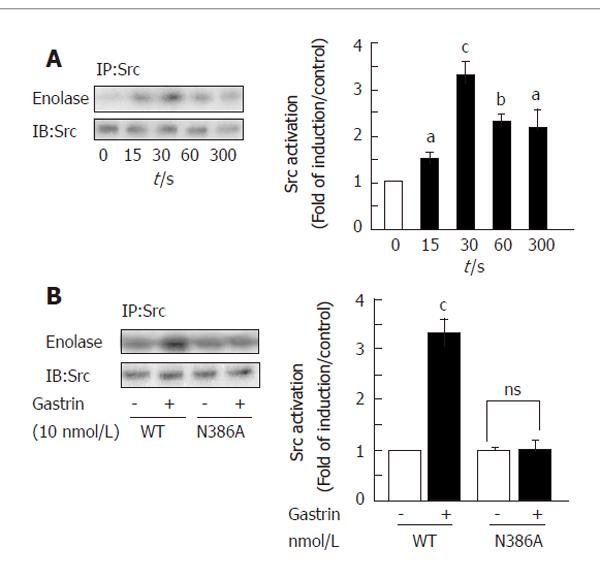
We first confirmed that the CCK2R was able to induce Src activation. In vitro tyrosine-kinase assays were performed in anti-Src immunoprecipitates from cell lysates containing equal amounts of protein. We detected a rapid and significant increase in Src activation 15 s after stimulation of the CCK2R by gastrin (Figure 3A).
In order to address the role of Src family kinases in proliferation of tumour acinar cells induced by CCK2R, we measured AR4-2J proliferation in the presence or absence of the Src specific inhibitor, PP2, 48 h after gastrin stimulation. CCK2R activation by gastrin induced a significant increase of cell proliferation. Treatment of cells with PP2 totally inhibited CCK2R-induced AR4-2J proliferation (Figure 3B).
We previously reported that CCK2R associates with the αq subunit of heterotrimeric G-proteins[1]. We therefore tested the hypothesis that Gαq might be involved in gastrin-induced Src activation.
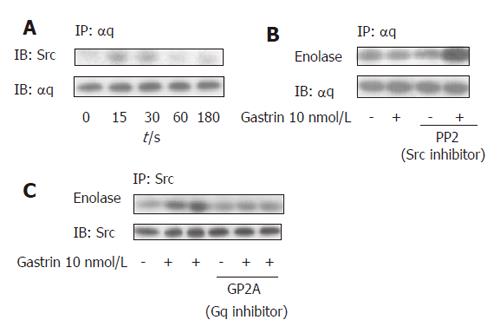
Src proteins were immunoprecipitated with specific antibodies and their association with Gαq was analyzed by western blot with an anti- αq antibody. An increase in the amount of Src proteins coprecipitated with Gαq was detected in response to gastrin (Figure 4A). This effect was time-dependent and the kinetic correlated with that of Src activation in this cellular model. We then examined whether Src-like tyrosine kinase activity was in association with Gαq following gastrin stimulation. An increase in tyrosine kinase activity was detected in anti- Gαq immunoprecipitates after gastrin stimulation which was abolished when samples were treated with the specific Src inhibitor, PP2 (Figure 4B). In addition, pretreatment of the AR42J cells with a specific Gαq inhibitor, GP2A, completely blocked the activation of Src by gastrin (Figure 4C).
To further investigate the molecular mechanisms involved in Src activation by the CCK2R we used COS-7 cells transiently transfected with cDNAs coding for the human wild type CCK2R (WT-CCK2R) or mutant CCK2R. We first validated this model for CCK2R-induced Src activation. Lysates from cells stimulated or not with gastrin were immunoprecipitated with anti-Src antibodies and kinase assays performed as described in methods. A rapid activation of Src (15 s), still detectable at 1 and 5 min, was found in response to Gastrin (Figure 5A). Western-blot analysis for Src protein expression revealed an equal amount of the protein in transfected cells.
Recently, we reported that the NPXXY motif (X represents any amino acid), located at the end of the 7th transmembrane domain of the CCK2R, was involved in Gq-dependent signaling pathways induced by the CCK2R[1,9]. In particular, mutation of the asparagine (N) into alanine (A) inhibits Gq-dependent pathways such as inositol triphosphate (IP3) production. We therefore tested the involvement of the NPXXY motif in Src activation by the CCK2R.
cDNAs coding for the WT receptor or the N386A mutant were transfected in COS-7 cells and Src activation was studied in response to Gastrin. Figure 5B clearly demonstrates that the CCK2R mutant cannot induce Src activation in response to Gastrin in contrast to the WT receptor.
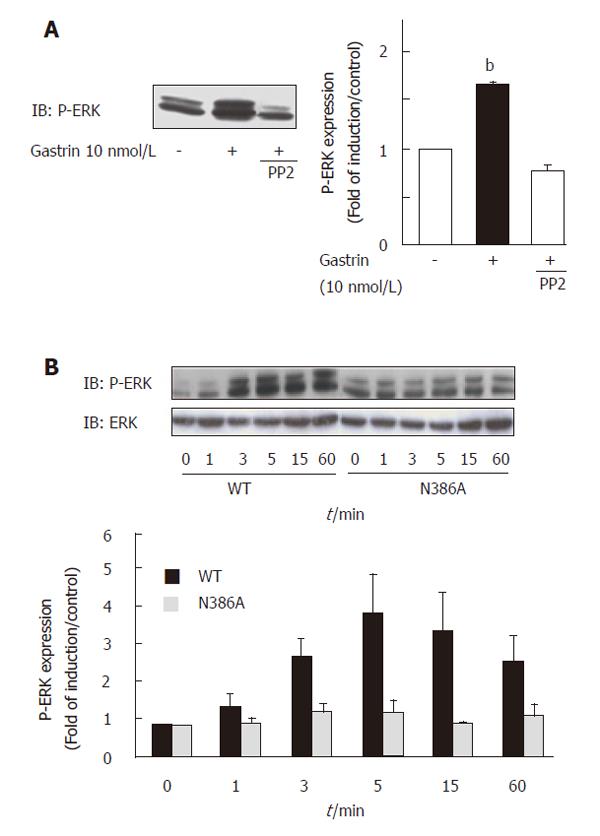
In addition, we demonstrated that ERK activation following gastrin stimulation was completely blocked by the Src inhibitor PP2, indicating that CCK2R-induced ERK activation was totally Src-dependent in this cellular model (Figure 6A). As expected, activation of the ERK pathway was also blocked when the CCK2R was mutated on the NPXXY motif (Figure 6B). Thus, our results demonstrated the involvement of the conserved NPXXY motif within the receptor sequence in the activation of the Src/ERK pathway by the CCK2R, and suggested putative role of Gq in this mechanism.
It is well demonstrated that numerous G protein-coupled receptors activate Src family kinases. However, very few publications have described the molecular mechanisms involved in Src activation by this receptor family. In the present study our results demonstrate that CCK2R-induced Src activation requires the NPXXY motif, and suggest a putative role of Gq in this mechanism. Indeed, this motif was previously described in Gq-dependent signalling pathways[1,9]. In addition, we showed that Src and Gαq are within one protein complex and we observed a Src-like tyrosine activity associated with Gq. However, despite the appearance of Src and Gαq in the same immunocomplex, we cannot exclude the possibility that Src activation by Gαq requires other protein. Our study also reveals that downstream of Src, activation of the ERK pathway by the CCK2R also requires the NPXXY motif.
The direct interaction of the Src SH3 domain with proline-rich motifs has been reported for the β3 adrenergic and the purinergic P2Y2 receptors[13,14]. Src family kinases also possess an SH2 domain that could directly bind to tyrosine-phosphorylated β2 adrenergic receptor[15]. We tested these two hypotheses in Src activation by the CCK2R. Indeed, the CCK2R has two proline-rich sequences which might interact with SH3 domains. In addition, it also has an ITIM-like motif within the C-terminus tail (LSYTTI) that is potentially phosphorylated on tyrosine residues, in turn leading to the recruitment of PLCγ hrough its SH2 domain[16]. Mutation of the prolines into serines, as well as the replacement of the tyrosine 438 of the ITIM-like motif by a phenylalanine did not affect the activation of Src by the CCK2R in COS-7 cells (data not shown).
Transactivation of receptor tyrosine kinases is another mechanism by which G protein-coupled receptors activate Src family kinases. Recently, the CCK2R has been shown to transactivate the EGF receptor[17-19]. However, this mechanism seems to be dependent on the cellular model. In the present study, we have observed that inhibition of the EGF receptor by AG1487 did not abolish CCK2R-mediated Src activation in AR42J and COS7 cells (data not shown).
Our study also shows that Src is activated upstream of the ERK pathway by the CCK2R in pancreatic tumor cells and contributes to the proliferative effects mediated by this receptor.
The development of cancer is thought to be dependent on the deregulation of normal signaling pathways involved in cell proliferation, thus conferring a growth advantage to cells. The role of Src and the ERK pathway in the regulation of cell growth is well documented. These signalling molecules have been implicated in the proliferative effects induced by tyrosine kinase receptors, cytokine receptors and G protein-coupled receptors. Activation of Src and ERK proteins has been observed in several human cancers and may contribute to the neoplastic phenotype. However, there is currently very little information regarding the role of the SRC/ERK pathway in the early stages of pancreatic carcinogenesis.
Here we report that the expression of the CCK2R in mouse pancreatic acinar tissue leads to strong activation of the Src tyrosine kinase and the ERK pathway. These transgenic mice display an increased growth of the pancreas and preneoplastic lesions. They also develop pancreatic tumors with a ductal phenotype similar to what is observed in human pancreatic cancers. Deregulation of the Src/ERK pathway by the CCK2R in these transgenic mice might represent an early step that contributes to cell proliferation, formation of preneoplastic lesions and pancreatic tumor development. In addition, while several studies have reported the in vitro activation of Src and ERKs by gastrin[10,11,20], this study is the first to demonstrate in vivo the up-regulation of the Src/ERK pathway by CCK2R.
In summary, our study describes the mechanism by which the CCK2R, a GPCR mainly coupled to Gq, activates Src. Our results show the involvement of the NPXXY motif within the receptor sequence in this activation, and suggest the putative role of Gq in this mechanism. Moreover, in pancreatic tumoral models we demonstrate in vitro and in vivo that the CCK2R activates the Src/ERK signaling pathway, a transduction cascade upregulated during tumorigenesis in human.
S- Editor Wang J L- Editor Zhu LH E- Editor Ma N
| 1. | Galés C, Kowalski-Chauvel A, Dufour MN, Seva C, Moroder L, Pradayrol L, Vaysse N, Fourmy D, Silvente-Poirot S. Mutation of Asn-391 within the conserved NPXXY motif of the cholecystokinin B receptor abolishes Gq protein activation without affecting its association with the receptor. J Biol Chem. 2000;275:17321-17327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Aly A, Shulkes A, Baldwin GS. Gastrins, cholecystokinins and gastrointestinal cancer. Biochim Biophys Acta. 2004;1704:1-10. [PubMed] |
| 3. | Clerc P, Leung-Theung-Long S, Wang TC, Dockray GJ, Bouisson M, Delisle MB, Vaysse N, Pradayrol L, Fourmy D, Dufresne M. Expression of CCK2 receptors in the murine pancreas: proliferation, transdifferentiation of acinar cells, and neoplasia. Gastroenterology. 2002;122:428-443. [RCA] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Wagner M, Luhrs H, Kloppel G, Adler G, Schmid RM. Malignant transformation of duct-like cells originating from acini in transforming growth factor transgenic mice. Gastroenterology. 1998;115:1254-1262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 142] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 5. | Grippo PJ, Nowlin PS, Demeure MJ, Longnecker DS, Sandgren EP. Preinvasive pancreatic neoplasia of ductal phenotype induced by acinar cell targeting of mutant Kras in transgenic mice. Cancer Res. 2003;63:2016-2019. [PubMed] |
| 6. | Coppola D. Molecular prognostic markers in pancreatic cancer. Cancer Control. 2000;7:421-427. [PubMed] |
| 7. | Yezhelyev MV, Koehl G, Guba M, Brabletz T, Jauch KW, Ryan A, Barge A, Green T, Fennell M, Bruns CJ. Inhibition of SRC tyrosine kinase as treatment for human pancreatic cancer growing orthotopically in nude mice. Clin Cancer Res. 2004;10:8028-8036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 97] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 8. | Ferrand A, Bertrand C, Portolan G, Cui G, Carlson J, Pradayrol L, Fourmy D, Dufresne M, Wang TC, Seva C. Signaling pathways associated with colonic mucosa hyperproliferation in mice overexpressing gastrin precursors. Cancer Res. 2005;65:2770-2777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Ferrand A, Kowalski-Chauvel A, Bertrand C, Escrieut C, Mathieu A, Portolan G, Pradayrol L, Fourmy D, Dufresne M, Seva C. A novel mechanism for JAK2 activation by a G protein-coupled receptor, the CCK2R: implication of this signaling pathway in pancreatic tumor models. J Biol Chem. 2005;280:10710-10715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Daulhac L, Kowalski-Chauvel A, Pradayrol L, Vaysse N, Seva C. Src-family tyrosine kinases in activation of ERK-1 and p85/p110-phosphatidylinositol 3-kinase by G/CCKB receptors. J Biol Chem. 1999;274:20657-20663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 90] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Seva C, Kowalski-Chauvel A, Blanchet JS, Vaysse N, Pradayrol L. Gastrin induces tyrosine phosphorylation of Shc proteins and their association with the Grb2/Sos complex. FEBS Lett. 1996;378:74-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Seva C, Scemama JL, Bastié MJ, Pradayrol L, Vaysse N. Lorglumide and loxiglumide inhibit gastrin-stimulated DNA synthesis in a rat tumoral acinar pancreatic cell line (AR42J). Cancer Res. 1990;50:5829-5833. [PubMed] |
| 13. | Cao W, Luttrell LM, Medvedev AV, Pierce KL, Daniel KW, Dixon TM, Lefkowitz RJ, Collins S. Direct binding of activated c-Src to the beta 3-adrenergic receptor is required for MAP kinase activation. J Biol Chem. 2000;275:38131-38134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 162] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 14. | Liu J, Liao Z, Camden J, Griffin KD, Garrad RC, Santiago-Pérez LI, González FA, Seye CI, Weisman GA, Erb L. Src homology 3 binding sites in the P2Y2 nucleotide receptor interact with Src and regulate activities of Src, proline-rich tyrosine kinase 2, and growth factor receptors. J Biol Chem. 2004;279:8212-8218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 136] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 15. | Fan G, Shumay E, Malbon CC, Wang H. c-Src tyrosine kinase binds the beta 2-adrenergic receptor via phospho-Tyr-350, phosphorylates G-protein-linked receptor kinase 2, and mediates agonist-induced receptor desensitization. J Biol Chem. 2001;276:13240-13247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 81] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Arnould M, Tassa A, Ferrand A, Archer E, Estève JP, Pénalba V, Portolan G, Escherich A, Moroder L, Fourmy D. The G-protein-coupled CCK2 receptor associates with phospholipase Cgamma1. FEBS Lett. 2004;568:89-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Guo YS, Cheng JZ, Jin GF, Gutkind JS, Hellmich MR, Townsend CM Jr. Gastrin stimulates cyclooxygenase-2 expression in intestinal epithelial cells through multiple signaling pathways. Evidence for involvement of ERK5 kinase and transactivation of the epidermal growth factor receptor. J Biol Chem. 2002;277:48755-48763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 78] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 18. | Miyazaki Y, Shinomura Y, Tsutsui S, Zushi S, Higashimoto Y, Kanayama S, Higashiyama S, Taniguchi N, Matsuzawa Y. Gastrin induces heparin-binding epidermal growth factor-like growth factor in rat gastric epithelial cells transfected with gastrin receptor. Gastroenterology. 1999;116:78-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 94] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 19. | Sinclair NF, Ai W, Raychowdhury R, Bi M, Wang TC, Koh TJ, McLaughlin JT. Gastrin regulates the heparin-binding epidermal-like growth factor promoter via a PKC/EGFR-dependent mechanism. Am J Physiol Gastrointest Liver Physiol. 2004;286:G992-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Daulhac L, Kowalski-Chauvel A, Pradayrol L, Vaysse N, Seva C. Gastrin stimulates the formation of a p60Src/p125FAK complex upstream of the phosphatidylinositol 3-kinase signaling pathway. FEBS Lett. 1999;445:251-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |