Published online Jul 14, 2006. doi: 10.3748/wjg.v12.i26.4253
Revised: December 1, 2005
Accepted: December 7, 2005
Published online: July 14, 2006
Autoimmune manifestations are common both in patients chronically infected by hepatitis C virus, and in patients transplanted for non-autoimmune diseases. A correlation between interferon based treatment and autoimmune diseases or the development of autoantibodies is well established in non-transplanted patients, but few data are available about transplanted patients. It is unclear whether interferon may increase the incidence of acute cellular rejection and there are few reports on the development of atypical autoimmune manifestations during post-liver transplantation interferon or pegylated interferon treatment. We describe a case of systemic lupus erythematosus following treatment with pegylated interferon alfa-2b in a transplanted patient with recurrence of chronic hepatitis C. Our experience suggest that pegylated interferon may induce autoimmune diseases in the immunosuppressed host, different from acute cellular rejection and call for a great attention to possible autoimmune disorders development during interferon based treatments in liver transplanted patients.
- Citation: Lodato F, Tamé MR, Colecchia A, Racchini C, Azzaroli F, D’Errico A, Casanova S, Pinna A, Roda E, Mazzella G. Systemic lupus erythematosus following virological response to peginterferon alfa-2b in a transplanted patient with chronic hepatitis C recurrence. World J Gastroenterol 2006; 12(26): 4253-4255
- URL: https://www.wjgnet.com/1007-9327/full/v12/i26/4253.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i26.4253
Autoimmune manifestations are common in patients chronically infected by hepatitis C virus (HCV)[1]. On the other hand, tissue autoantibodies are common in liver recipients transplanted for non autoimmune diseases and may be associated with negative graft outcome[2,3]. The safety and efficacy of interferon (IFNs) and the newest pegylated interferons (Peg-IFNs) for the treatment of recurrent hepatitis C in transplanted patients are still debated[4,5]. In particular, it is unclear whether IFN may increase the incidence of acute cellular rejection (ACR) and there are no reports on the development of atypical autoimmune manifestations during post-liver transplantation (LT) IFN or Peg-IFN treatment.
We report a case of severe autoimmune disease, different from ACR, during treatment with Peg-IFN alfa-2b in a transplanted patient with recurrence of chronic hepatitis C (CHC).
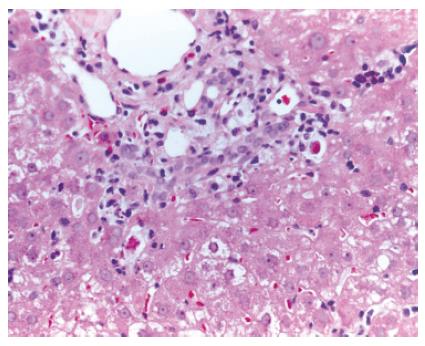
A 55-year-old man, underwent LT in March 2001 for HCV genotype 1 liver related cirrhosis. Acute immunosuppressive (IS) schedule was cyclosporine, azathioprine (AZA) and steroids. According to the Transplantation Unit IS protocol, AZA and steroids were stopped 3 wk and 1 year after LT, respectively. Screening tests for LT revealed the presence of cryoglobulins with a cryocrite of 8% and antinuclear antibodies (ANA) at low titre (1/160) with homogeneous pattern. After LT, clinical outcome was regular until January 2002, when the patient showed a persistent mild increase of transaminases (ALT 115 U/L and AST 103 U/L) with high viral load (17.5 MEq/mL, Versant HCV-RNA 3.0 bDNA, Bayer). Liver histology showed mildly active chronic hepatitis with severe fibrosis, presence of lymphocytes and macrovescicular steatosis, suggestive of HCV recurrence (Figure 1).
In October 2002 the patient started a cycle of Peg-IFN alfa-2b (1.1 mcg/kg per week) and Ribavirin (6.4 mg/kg per day). After 4 wk of treatment transaminases were normal. HCV-RNA showed a 2 log fall (0.01 MEq/mL) at wk 12; became undetectable by branched DNA, but still positive by polymerase chain reaction (TMA test, Versant HCV-RNA, Bayer) at wk 24 and finally negative by PCR at wk 36.
At wk 44 the patient presented migrant arthritis and the following biochemical parameters: normal transaminases, CyA 240 ng/mL, increased gamma-glutamyltransferase (γGT), alkaline phosphatase (ALP) and bilirubin (384 U/L, 690 U/L and 1.69 mg/dL, respectively), gamma-globulins 30%, Waaler-Rose 1/1280, ANA 1/640 and anti-DNA positive. No vascular or biliary complications were revealed by ultrasound and computed tomography, nor any signs of infectious diseases were present. Suspicion of an immune mediated manifestation, prednisone 10 mg/d was started. However, despite the presence of signs of autoimmunity we decided to complete the Peg-IFN cycle in consideration of the fact that we were almost at the end of the planned 48 wk of treatment with the patient responding to Peg-IFN.
At wk 48 the patient was asymptomatic, transaminases and bilirubin were normal, HCV-RNA negative by PCR, while ALP and γGT were decreased (ALP 350 U/L and γGT 94 U/L). Peg-IFN was stopped and steroids were maintained.
One month later, the patient developed pleuro-pericardial effusion and ascites. Liver function tests (LFTs) were normal, HCV-RNA was negative (PCR) and CyA within the therapeutic range; ANA was very high (1/1280) as well as perinuclear anti-neutrophil cytoplasmatic antibodies (pANCA) (1:320). Therefore, our patient developed a syndrome characterised by high titre autoantibodies, migrant arthritis and serositis. Following the current criteria, the diagnosis of systemic lupus erythematosus could have been made[6,7]. Prednisone was increased to 15 mg/d and diuretics introduced, resulting in the remission of liquid effusions and a progressive reduction of autoantibodies (ANA 1:160 and pANCA negative). We did not increase further the prednisone dosage in consideration of the possibility of an HCV re-activation in an already immunosuppressed patient and because of the initial clinical remission of autoimmune manifestations.
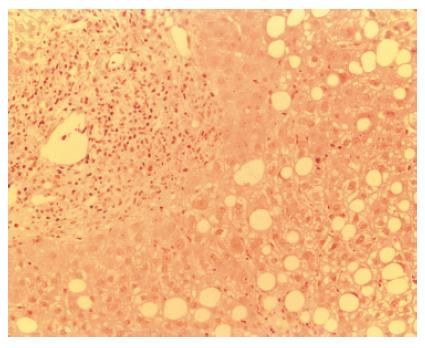
In March 2004, four months later, the patient presented an important increase of all LFT (GOT 257 U/L, GPT 197 U/L, ALP 438 U/L, γGT 356 U/L, total bilirubin 20.35 mg/dL, with direct bilirubin 17.20 mg/dL) with normal eosinophils, serum HCV-RNA positive at low titre (2.96 MEq/mL). CyA was close to the lower limit of the therapeutic range (100 ng/mL). Liver biopsy revealed the presence of interface hepatitis, numerous rosettes, cholangitis with ductular proliferation and biliocytes regression (Figure 2). Histological findings were not suggestive for HCV recurrence and did not fulfil the standard criteria for acute or chronic rejection[8]. Therefore, International Criteria for Autoimmune Hepatitis (AIH score)[9] were applied, according to which the patient was categorized as positive for “probable AIH” (score + 11). Consequently, steroid treatment was increased and the patient was switched from cyclosporine to tacrolimus. Response to immunosuppressive treatment was slow but progressive until normalization of LFTs.
It is well known that treatment with IFN may cause autoimmune diseases or the development of autoantibodies in non transplanted patients[10-13] while few data are available on transplanted ones. In particular, to the best of our knowledge, it has never been described the development of SLE after LT during interferon treatment. Only two cases of SLE have been described during IFN treatment in immunocompetent patients treated for HCV infection[12]. In a series of 677 patients treated for HCV infection, 4% developed autoimmune diseases, the majority (62%) being represented by thyroid dysfunction; only 1 patient developed an SLE-like syndrome with an incidence of 0.15% in the study population[14]. Alfa-IFN therapy has been moreover associated with the development of idiopathic thrombocytopenic purpura, myasthenia gravis, Addison’s disease, diabetes, COELIAC disease, rheumatoid arthritis, primary biliary cirrhosis, polymiositis, psoriasis[12,15-23].
Antiviral actions of alfa-IFN include the induction of several proteins, such as protein kinase (PKR) and 2’,5’-oligoadenylate synthetase, important in viral dynamics[24]. Moreover, IFNs induce the expression of MHC both on antigen presenting cells (APC) and hepatocytes, resulting in a virus-specific lysis of infected cells mediated by cytotoxic T-cell response[25]. Thus, IFNs may lead to the development of autoimmune diseases by the up-regulation of MHC in both transplanted and non transplanted patients. Moreover, the interaction of IFN activities in a particular pathway, such as in the immunosuppressed host, may lead to severe autoimmune manifestations that can compromise the graft survival. A relation between virological response and ACR was suggested in a recent study. Authors supposed that viral eradication improves microsomial function leading to a decrease of IS drugs levels[4]. The same mechanism may play a role in the induction of other autoimmune diseases such as that occurred to our patient who developed an SLE. Furthermore, our patient was on treatment with calcineurin inhibitors that have been thought to predispose to autoimmunity by interfering with T cell maturation and developing autoaggressive T-cells clones[26]. It is therefore possible that various mechanisms contribute to the development of autoimmune manifestations after liver transplantation.
In conclusion, the clinician should be aware of the possible development of autoimmune disorders, different from ACR, during interferon based treatment in LT patients, especially if signs of autoimmunity are present before starting IFN.
S- Editor Wang J L- Editor Zhu LH E- Editor Bai SH
| 1. | Clifford BD, Donahue D, Smith L, Cable E, Luttig B, Manns M, Bonkovsky HL. High prevalence of serological markers of autoimmunity in patients with chronic hepatitis C. Hepatology. 1995;21:613-619. [PubMed] |
| 2. | Kerkar N, Hadzić N, Davies ET, Portmann B, Donaldson PT, Rela M, Heaton ND, Vergani D, Mieli-Vergani G. De-novo autoimmune hepatitis after liver transplantation. Lancet. 1998;351:409-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 264] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 3. | Conti F, Dousset B, Levillayer H, Gruska I, Weill B, Calmus Y. Autoantibodies after liver transplantation: a marker of allograft disease. J Hepatol. 1997;26:150A. |
| 4. | Kugelmas M, Osgood MJ, Trotter JF, Bak T, Wachs M, Forman L, Kam I, Everson GT. Hepatitis C virus therapy, hepatocyte drug metabolism, and risk for acute cellular rejection. Liver Transpl. 2003;9:1159-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 54] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Féray C, Samuel D, Gigou M, Paradis V, David MF, Lemonnier C, Reynès M, Bismuth H. An open trial of interferon alfa recombinant for hepatitis C after liver transplantation: antiviral effects and risk of rejection. Hepatology. 1995;22:1084-1089. [PubMed] [DOI] [Full Text] |
| 6. | Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, Schaller JG, Talal N, Winchester RJ. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271-1277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9555] [Cited by in RCA: 10026] [Article Influence: 233.2] [Reference Citation Analysis (0)] |
| 7. | Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7731] [Cited by in RCA: 8646] [Article Influence: 308.8] [Reference Citation Analysis (0)] |
| 8. | Banff schema for grading liver allograft rejection: an international consensus document. Hepatology. 1997;25:658-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1016] [Cited by in RCA: 1001] [Article Influence: 35.8] [Reference Citation Analysis (1)] |
| 9. | Alvarez F, Berg PA, Bianchi FB, Bianchi L, Burroughs AK, Cancado EL, Chapman RW, Cooksley WG, Czaja AJ, Desmet VJ. International Autoimmune Hepatitis Group Report: review of criteria for diagnosis of autoimmune hepatitis. J Hepatol. 1999;31:929-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2003] [Cited by in RCA: 1985] [Article Influence: 76.3] [Reference Citation Analysis (0)] |
| 10. | Fattovich G, Giustina G, Favarato S, Ruol A. A survey of adverse events in 11,241 patients with chronic viral hepatitis treated with alfa interferon. J Hepatol. 1996;24:38-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 323] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 11. | Durelli L, Ferrero B, Oggero A, Verdun E, Ghezzi A, Montanari E, Zaffaroni M. Thyroid function and autoimmunity during interferon beta-1b treatment: a multicenter prospective study. J Clin Endocrinol Metab. 2001;86:3525-3532. [PubMed] [DOI] [Full Text] |
| 12. | Wilson LE, Widman D, Dikman SH, Gorevic PD. Autoimmune disease complicating antiviral therapy for hepatitis C virus infection. Semin Arthritis Rheum. 2002;32:163-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 121] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 13. | Fabbri C, Jaboli MF, Giovanelli S, Azzaroli F, Pezzoli A, Accogli E, Liva S, Nigro G, Miracolo A, Festi D. Gastric autoimmune disorders in patients with chronic hepatitis C before, during and after interferon-alpha therapy. World J Gastroenterol. 2003;9:1487-1490. [PubMed] |
| 14. | Okanoue T, Sakamoto S, Itoh Y, Minami M, Yasui K, Sakamoto M, Nishioji K, Katagishi T, Nakagawa Y, Tada H. Side effects of high-dose interferon therapy for chronic hepatitis C. J Hepatol. 1996;25:283-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 280] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 15. | Aspinall RJ, Pockros PJ. The management of side-effects during therapy for hepatitis C. Aliment Pharmacol Ther. 2004;20:917-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 35] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, Koury K, Ling M, Albrecht JK. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4736] [Cited by in RCA: 4558] [Article Influence: 189.9] [Reference Citation Analysis (0)] |
| 17. | Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Gonçales FL, Häussinger D, Diago M, Carosi G, Dhumeaux D. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4847] [Cited by in RCA: 4747] [Article Influence: 206.4] [Reference Citation Analysis (0)] |
| 18. | Hadziyannis SJ, Sette H Jr, Morgan TR, Balan V, Diago M, Marcellin P, Ramadori G, Bodenheimer H Jr, Bernstein D, Rizzetto M. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med. 2004;140:346-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2216] [Cited by in RCA: 2109] [Article Influence: 100.4] [Reference Citation Analysis (0)] |
| 19. | McHutchison JG, Gordon SC, Schiff ER, Shiffman ML, Lee WM, Rustgi VK, Goodman ZD, Ling MH, Cort S, Albrecht JK. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. Hepatitis Interventional Therapy Group. N Engl J Med. 1998;339:1485-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2509] [Cited by in RCA: 2434] [Article Influence: 90.1] [Reference Citation Analysis (0)] |
| 20. | Davis GL, Esteban-Mur R, Rustgi V, Hoefs J, Gordon SC, Trepo C, Shiffman ML, Zeuzem S, Craxi A, Ling MH. Interferon alfa-2b alone or in combination with ribavirin for the treatment of relapse of chronic hepatitis C. International Hepatitis Interventional Therapy Group. N Engl J Med. 1998;339:1493-1499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 927] [Cited by in RCA: 895] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 21. | McHutchison JG, Manns M, Patel K, Poynard T, Lindsay KL, Trepo C, Dienstag J, Lee WM, Mak C, Garaud JJ. Adherence to combination therapy enhances sustained response in genotype-1-infected patients with chronic hepatitis C. Gastroenterology. 2002;123:1061-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 750] [Cited by in RCA: 736] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 22. | Fried MW. Side effects of therapy of hepatitis C and their management. Hepatology. 2002;36:S237-S244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 320] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 23. | Dieperink E, Willenbring M, Ho SB. Neuropsychiatric symptoms associated with hepatitis C and interferon alpha: A review. Am J Psychiatry. 2000;157:867-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 290] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 24. | Zeuzem S, Schmidt JM, Lee JH, Rüster B, Roth WK. Effect of interferon alfa on the dynamics of hepatitis C virus turnover in vivo. Hepatology. 1996;23:366-371. [PubMed] |
| 25. | Samuel CE. Antiviral actions of interferons. Clin Microbiol Rev. 2001;14:778-809, table of contents. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2029] [Cited by in RCA: 2000] [Article Influence: 83.3] [Reference Citation Analysis (0)] |
| 26. | Damoiseaux JG, van Breda Vriesman PJ. Cyclosporin A-induced autoimmunity: the result of defective de novo T-cell development. Folia Biol (Praha). 1998;44:1-9. [PubMed] |