Published online Jul 14, 2006. doi: 10.3748/wjg.v12.i26.4228
Revised: February 14, 2006
Accepted: February 28, 2006
Published online: July 14, 2006
AIM: To investigate the distribution of beta-catenin in nuclei or membrane/cytoplasm of gastric carcinoma cells, the relationship between E-cadherin gene methylation and its expression, and the role of beta-catenin and E-cadherin as potential molecular markers in predicting tumor infiltration.
METHODS: Twenty-nine cases of gastric carcinoma, classified as diffuse and intestinal variants, were selected for study. Nuclear and cytoplasmic proteins were purified and beta-catenin content was detected by ELISA. DNA methylation of E-cadherin/CDH1 gene promoter was studied by methylation-specific PCR and compaired with E-cadherin expression detected by immunohistochemistry.
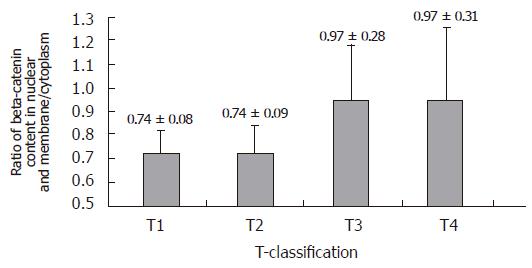
RESULTS: In 27 cases of gastric carcinoma, the ratio of beta-catenin content between nuclei and membrane/cytoplasm was correlated with the T-classification (r = 0.392, P = 0.043). The significance was present between T2 and T3 groups. No correlation was detected between diffuse and intestinal variants in terms of their beta-catenin distribution. In 21 cases of diffuse variants of gastric carcinoma, there was a difference in E-cadherin expression between CDH1 gene-methylated group and non-methylated group (29 % vs 71 %, P = 0.027). No correlation between CDH1 gene methylation and T-classification was found, neither was the significance between E-cadherin expression and tumor infiltration grade.
CONCLUSION: Comparative analysis of nuclear and membrane/cytoplasmic beta-catenin can predict local tumor infiltration. E-cadherin/CDH1 gene methylation is an important cause for its gene silence in diffuse variant gastric carcinoma. Methylation of CDH1 gene in the absence of E-cadherin is an early event in gastric carcinogenesis.
- Citation: Wang L, Zhang F, Wu PP, Jiang XC, Zheng L, Yu YY. Disordered beta-catenin expression and E-cadherin/CDH1 promoter methylation in gastric carcinoma. World J Gastroenterol 2006; 12(26): 4228-4231
- URL: https://www.wjgnet.com/1007-9327/full/v12/i26/4228.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i26.4228
Gastric carcinoma is highly malignant and usually results in a poor outcome. Until now there is no satisfactory tumor marker for predicting its evolution. E-cadherin, a transmembrane glycoprotein of 120 KD which is expressed in normal epithelium, may play an important role both in cell-cell adhesion and in tumor invasion and metastasis. The low expression of E-cadherin may favor the dissociation of carcinoma cells from one another for their invading out of basal membrane. Though mutation and allelic loss have been confirmed as major mechanisms for E-cadherin (CDH1) gene inactivation in many malignancies[1], it has been recently reported that CDH1 promoter methylation could be frequently detected in gastric carcinoma[2]. Still it is controversial whether DNA methylation is the main cause for E-cadherin/CDH1 gene silence. As a copartner of E-cadherin, beta-catenin is critical for intercellular adhesion in membrane and cytoplasm of cells, it also plays a role as a transcription activating protein in nuclei[3]. The nuclear accumulation of beta-catenin may stimulate gastric epithelium proliferation[4], nevertheless the effect of beta-catenin on tumor infiltration in gastric carcinoma is waiting to be more precisely studied by quantitative analysis. The aim of the present study was to investigate the relationship between E-cadherin gene methylation and its expression, the distribution of beta-catenin in nuclei and cytoplasm in gastric carcinoma, and the role of beta-catenin and E-cadherin as potential molecular markers in predicting tumor infiltration.
Tissue blocks were obtained from the Department of Digestive Disease, Shanghai Ruijing Hospital and the Department of Pathology, Shanghai No.2 Hospital, involving 29 cases of gastric carcinoma operated from 2002 to 2003, of which 21 were diffuse variants and 8 were intestinal variants, together with 5 paraneoplastic non-tumor gastric tissues. The samples were freshly frozen at -70°C for DNA and protein extraction. Also the samples were fixed in 40 g/L formalin buffer then paraffin-embedded routinely for immunohistochemistry. T-classification revealed that 3 cases were T1, 5 T2, 18 T3 and 3 T4.
Nuclear and cytoplasmic protein was isolated for beta-catenin analysis. Frozen tumor tissue (1.0 cm × 1.0 cm × 1.0 cm) from each case was cut into minimal sections and homogenized manually for 5 min at 4°C with 700 μL cytoplasmic lysis buffer (0.15 mol/L NaCl, 10 mmol/L HEPES, 1mmol/L EDTA, 6 mL/L NP-40). Membrane and cytoplasmic lysis were checked by microscopic examination. The nuclei were collected by centrifuging for 5 min at 1 300 r/min at 4°C, then vigorously homogenized for 30 min at 4°C with 500 μL nuclear lysis buffer (0.4 mol/L NaCl, 20 mmol/L HEPES, 0.2 mmol/L EDTA, 0.5 mmol/L PMSF, 250 mL/L glycerol, 1.2 mmol/L MgCl2, 0.5 mmol/L DTT, 0.5 mg/L leupeptin, 0.5 mg/L aprotinin, 0.5 mg/L pepstatin). The protein in nuclear or cytoplasmic solution was tested by Coomassie reagents following the manufacturer's instructions (Coomassie Plus-200 protein assay reagent, No-23238, Hyclone-PIERCE, USA).
Nuclear or cytoplasmic content of beta-catenin was analyzed by ELISA. The proteins were immobilized onto 96-well microtiter plates at 4°C, and washed with PBS-0.5 mL/L Tween 20. Monoclonal mouse antibody (anti-beta-catenin, M-0545, Antibody Diagnostica Company, USA) was applied in 1:25 dilution of PBS-0.5 mL/L Tween 20 at 37°C for 1h. After being washed, the wells were incubated with AKP-conjugated secondary antibody and then washed again and 1mg/mL pNPP was added. Absorbance of eluted dye was measured at A312nm. Negative controls were performed by replacing primary antibody with PBS-0.5 mL/L Tween 20.
For each sample, the frozen gastric tissue (0.5 cm × 0.5 cm × 0.5 cm) was cut into minimal sections and incubated with proteinase K (20 g/L) at 55°C for 24 h. The DNA was extracted by standard phenol/chloroform technique.
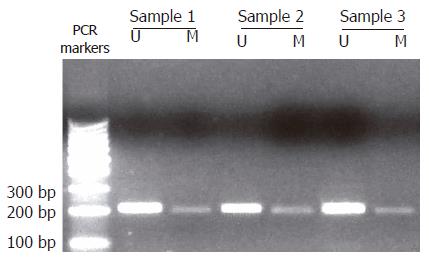
DNA samples (100 μL) were incubated in 0.2 mol/L NaOH at 37°C for 10 min, then modified with sodium bisulfite solution following the manufacturer's instructions (CpGenome DNA modification kit, S7820, Ingergen Company). The modified DNA was amplified with E-cadherin/CDH1 gene-specific primers as follow: methylated-specific primer set: sense 5'-GGTGAATTTTTAGTTAATTAGCCGGTAC-3' and antisense 5'-CATAACTAACCG AAAACGCCG-3', yielding a product of 204 bp; unmethylated-specific primer set: sense 5'-GGTAGGTGAATTTTTAGTTAATTAGTGGTA-3' and antisense 5'-ACCCATAACTAACCAAAAACACCA-3', yielding a product of 211 bp[5]. The PCR mixture (50 μL in total) contained 1 × buffer (SABC Biochemical) with 1.5 mmol/L MgCl2, 0.2 mmol dNTPs, 0.2 μmol of each primer, and 4 μL of DNA sample. PCR conditions were 10 min at 94°C, after which 3U of Taq DNA polymerase (SABC Biochemical) was added, and 35 cycles at 94°C for 50 s, at 57°C for 40 s, at 72°C for 90 s, and a final extension at 72°C for 5 min. The positive control was performed on DNA from normal gastric tissue by using unmethylated-specific primers, and the negative control was prepared on PCR mixture without primers. The PCR products were migrated by electrophoresis on 20 g/L agarose gel, with 100 bp DNA ladder as a DNA marker.
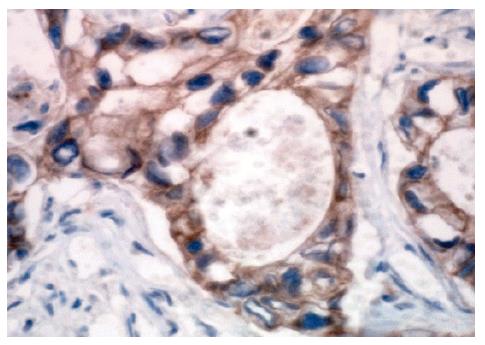
Formalin-fixed paraffin-embedded tissue sections (4 µm thick) were deparaffinized in xylene for 10 min, and rehydrated through graded alcohols to water. Antigen retrieval was performed by microwave of tissue sections in 10 mmol/L sodium citrate buffer (pH 6.0) for 15 min at 750 W. Endogenous peroxidase activity was blocked with 3 mL/L hydrogen peroxide. Primary antibody (anti-E-cadherin, mouse monoclonal antibody, Antibody Diagnostic Company, USA) was applied at 1:25 dilution and incubated for 1 h at room temperature. The slides were washed in PBS for 15 min, and secondary incubations were carried out by using anti-mouse antibody-polymerized dextran-HRP complex (ADI Two-Step System, Antibody Diagnostica Company, USA) for 30 min. Immunoreactivity was visualized with 3,3'-diaminobenzidine tetrahydrochloride (DAB), and counterstained with hematoxylin. The brown-stained color in cell membrane by DAB was defined as positive reactivity. Negative controls were performed by replacing primary antibody with PBS. The DAB staining in nuclei or cytoplasm was considered as negative.
The expression of E-cadherin was considered as positive when at least 10% of tumor cells were colored by DAB[6]. In quantitative evaluation, 5 microscopic fields were analyzed on each tissue slide. The percentage of positive cells (300 to 900 tumor cells counted for each sample) was classified as grade 1 (11%-25%), grade 2 (26%-50%), grade 3 (51%-75%) and grade 4 (more than 75%).
Fisher’s exact probability test was used to evaluate the relation between E-cadherin/CDH1 gene methylation and its expression. Correlations among the E-cadherin protein, the ratio of beta-catenin content between nuclei and membrane/cytoplasm, the tumor infiltration grading and the Lauren's typing were evaluated by method of Spearman (statistical software: SAS 6.12).
Twenty-seven cases of gastric carcinoma were successfully analyzed for beta-catenin distribution. The ratio of beta-catenin content between nuclei and membrane/cytoplasm was correlated with the T-classification (r = 0.392, P = 0.043). The significance was present between T2 and T3 groups (Figure 1). There was no relation between diffuse variant and intestinal variant in terms of their beta-catenin distribution in tumor cells.
In 29 cases of gastric carcinoma, CDH1 gene promoter methylation was identified in 13 cases (45%) (Figure 2), of which 7 cases were histologically diffuse variants (33%), 6 cases intestinal variants (75%). All 3 T1 cases were found to be positive for CDH1 gene methylation, so did 1 of 5 T2 cases, 7 of 18 T3 cases and 2 of 3 T4 cases. There was no difference in diffuse and intestinal variants for their CDH1 gene methylation, and no correlation between CDH1 gene methylation and T-classification was found. The CDH1 gene methylation was also identified in 3 specimens of paraneoplastic non-tumor gastric tissues.
E-cadherin expression was detected in 18 of 29 gastric carcinoma cases (62%) (Figure 3), of them 12 cases were diffuse variants (57%) and 6 cases intestinal variants (75%). Two of 3 T1 cases, 2 of 5 T2 cases, 11 of 18 T3 cases and all 3 T4 cases were found to be positive for E-cadherin expression.
Of the 13 gastric carcinoma cases which were identified for CDH1 gene methylation, E-cadherin expression was found in 2 of 7 diffuse variants and 5 of 6 intestinal variants. In 21 cases of diffuse variants, there was a difference in E-cadherin expression between CDH1 gene-methylated group and non-methylated group (29% vs 71%, P = 0.027). No significance between E-cadherin expression and T-classification was found, neither was the relation between diffuse variant and intestinal variant for their E-cadherin expression.
Our previous study demonstrated that E-cadherin and beta-catenin are co-expressed in gastric carcinoma[7]. The regulation at their transcriptional levels seems to be independently controlled by different mechanisms[8]. Beta-catenin is a bifunctional protein, location in nuclear or cytoplasm/membrane is critical for its activity as an adhesive factor or proto-oncoprotein. In nuclei, beta-catenin plays a role as a target of the wnt signaling pathway in stimulating cell proliferation through activation of cyclin D1[9] and c-myc gene[10]. The nuclear translocation of beta-catenin depends on its stabilization and the P13K signaling pathway. The latter may be activated by EB virus infection[11], which is also thought to be an important cause for E-cadherin/CDH1 gene methylation[12].
The nuclear accumulation of beta-catenin seems to be helpful, regarding tumor infiltration in gastric[13] and colorectal carcinoma[14], but this accumulation could also be found in early neoplasia or even in non-tumor metaplasia[4], and beta-catenin expression at cell level does not appear to change from normal to carcinoma epithelium[15]. Our results indicated that the evaluation of both nuclear and membrane/cytoplasmic beta-catenin expression might have a prognostic implication and a potential diagnostic value for gastric carcinoma. In the present study, we found a significant variation of beta-catenin distribution from T2 to T3 grade, suggesting that beta-catenin plays an important role in tumor infiltration in gastric smooth muscle. The possible mechanism may be that beta-catenin could regulate the matrix metalloproteinase activity in degradation of extracellular matrix components[16], and this regulation is mainly focused on local infiltration rather than metastasis[17].
E-cadherin is an indispensable protein for cell adhesion in normal epithelium. Loss of its function is propitious to tumor invasion. For silence of the E-cadherin/CDH1 gene in gastric carcinoma, recent studies showed that not only the changes of DNA sequence but also the epigenetic modification[18,19] such as DNA methylation, may play an important role in the loss of E-cadherin expression.
DNA methylation has attracted great attention recently, as many tumor suppresser genes contain CpG-rich promoters called CpG islands, which are normally unmethylated. Silence of these genes may occur in association with the aberrant hypermethylation of their promoter regions. It has been reported that E-cadherin/CDH1 gene is frequently methylated in colorectal and gastric carcinoma, but its endogenetic changes such as mutation are rare[12,20]. An in vitro study demonstrated that the E-cadherin expression, associated with the cell metamorphosis, could be induced by demethylation drugs[21]. These studies indicate that DNA methylation may be a possible mechanism for E-cadherin/CDH1 gene silence in human gastric carcinoma.
In the present study, 45% of gastric carcinomas were positive for E-cadherin/CDH1 gene methylation, which corresponds to the result of previous study[22]. We showed that E-cadherin expression was negatively correlated with its gene methylation in diffuse variants, indicating that DNA methylation may diminish E-cadherin expression and thus favor the devastating activity of the tumor. The data reported here do not provide evidence of correlation between CDH1 gene methylation and tumor infiltration, but are in agreement with the hypothesis that methylation status of CDH1 promoter may be an early event in gastric carcinogenesis[15].
In conclusion, comparative analysis of nuclear and membrane/cytoplasmic beta-catenin can predict tumor infiltration in gastric wall, and the E-cadherin/CDH1 gene methylation is an important cause for its gene silence in diffuse variant gastric carcinomas. Analysis for detecting distribution of beta-catenin protein and methylation state of E-cadherin/CDH1 gene helps to understand the dynamic process of oncogenesis.
The authors thank Dr. Jian-Min Tang (Department of Pathology, Shanghai No.2 Hospital) for his collaboration in acquiring the tumor samples and Professors Yan-Fei Zhao and Xing-Xu Du and Dr. Jian-Guo Dong for their kindly supports.
S- Editor Wang J L- Editor Wang XL E- Editor Liu Y
| 1. | Oda T, Kanai Y, Oyama T, Yoshiura K, Shimoyama Y, Birchmeier W, Sugimura T, Hirohashi S. E-cadherin gene mutations in human gastric carcinoma cell lines. Proc Natl Acad Sci USA. 1994;91:1858-1862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 217] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 2. | Graziano F, Arduini F, Ruzzo A, Mandolesi A, Bearzi I, Silva R, Muretto P, Testa E, Mari D, Magnani M. Combined analysis of E-cadherin gene (CDH1) promoter hypermethylation and E-cadherin protein expression in patients with gastric cancer: implications for treatment with demethylating drugs. Ann Oncol. 2004;15:489-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Cadigan KM, Nusse R. Wnt signaling: a common theme in animal development. Genes Dev. 1997;11:3286-3305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1965] [Cited by in RCA: 1971] [Article Influence: 70.4] [Reference Citation Analysis (0)] |
| 4. | Romiti A, Zullo A, Borrini F, Sarcina I, Hassan C, Winn S, Tomao S, Vecchione A, Morini S, Mingazzini P. Relationship between beta-catenin expression and epithelial cell proliferation in gastric mucosa with intestinal metaplasia. World J Gastroenterol. 2005;11:4400-4403. [PubMed] |
| 5. | Graff JR, Herman JG, Myöhänen S, Baylin SB, Vertino PM. Mapping patterns of CpG island methylation in normal and neoplastic cells implicates both upstream and downstream regions in de novo methylation. J Biol Chem. 1997;272:22322-22329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 226] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 6. | Shiozaki H, Iihara K, Oka H, Kadowaki T, Matsui S, Gofuku J, Inoue M, Nagafuchi A, Tsukita S, Mori T. Immunohistochemical detection of alpha-catenin expression in human cancers. Am J Pathol. 1994;144:667-674. [PubMed] |
| 7. | Wang L, Zhang F, Wu PP, Jiang XC, Zheng L, Yu YY. Relationgship between E-cadherin or β-catenin Expression and Gastric Carcinoma. J Shanghai Sec Medi Univ. 2005;25:675-678. |
| 8. | Lynch HT, Grady W, Suriano G, Huntsman D. Gastric cancer: new genetic developments. J Surg Oncol. 2005;90:114-133; discussion 133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 135] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 9. | Utsunomiya T, Doki Y, Takemoto H, Shiozaki H, Yano M, Sekimoto M, Tamura S, Yasuda T, Fujiwara Y, Monden M. Correlation of beta-catenin and cyclin D1 expression in colon cancers. Oncology. 2001;61:226-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 60] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Brabletz T, Herrmann K, Jung A, Faller G, Kirchner T. Expression of nuclear beta-catenin and c-myc is correlated with tumor size but not with proliferative activity of colorectal adenomas. Am J Pathol. 2000;156:865-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 118] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 11. | Morrison JA, Klingelhutz AJ, Raab-Traub N. Epstein-Barr virus latent membrane protein 2A activates beta-catenin signaling in epithelial cells. J Virol. 2003;77:12276-12284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 145] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 12. | Sudo M, Chong JM, Sakuma K, Ushiku T, Uozaki H, Nagai H, Funata N, Matsumoto Y, Fukayama M. Promoter hypermethylation of E-cadherin and its abnormal expression in Epstein-Barr virus-associated gastric carcinoma. Int J Cancer. 2004;109:194-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 57] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Song BJ, Park YJ, Kim HS, Kim CN, Chang SH. [Expression of beta-catenin and E-cadherin in early gastric cancer: correlation with clinicopathologic parameters]. Korean J Gastroenterol. 2004;43:82-89. [PubMed] |
| 14. | Wong SC, Lo ES, Chan AK, Lee KC, Hsiao WL. Nuclear beta catenin as a potential prognostic and diagnostic marker in patients with colorectal cancer from Hong Kong. Mol Pathol. 2003;56:347-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Mingchao TR, Stockton P, Sun K, Sills RC, Clayton N, Portier M, Flake G. Loss of E-cadherin expression in gastric intestinal metaplasia and later stage p53 altered expression in gastric carcinogenesis. Exp Toxicol Pathol. 2001;53:237-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Saeki H, Tanaka S, Sugimachi K, Kimura Y, Miyazaki M, Ohga T, Sugimachi K. Interrelation between expression of matrix metalloproteinase 7 and beta-catenin in esophageal cancer. Dig Dis Sci. 2002;47:2738-2742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Utsunomiya T, Doki Y, Takemoto H, Shiozaki H, Yano M, Inoue M, Yasuda T, Fujiwara Y, Monden M. Clinical significance of disordered beta-catenin expression pattern in human gastric cancers. Gastric Cancer. 2000;3:193-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Jones PA, Laird PW. Cancer epigenetics comes of age. Nat Genet. 1999;21:163-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1657] [Cited by in RCA: 1608] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 19. | Baylin SB, Herman JG. DNA hypermethylation in tumorigenesis: epigenetics joins genetics. Trends Genet. 2000;16:168-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1168] [Cited by in RCA: 1169] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 20. | Garinis GA, Menounos PG, Spanakis NE, Papadopoulos K, Karavitis G, Parassi I, Christeli E, Patrinos GP, Manolis EN, Peros G. Hypermethylation-associated transcriptional silencing of E-cadherin in primary sporadic colorectal carcinomas. J Pathol. 2002;198:442-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Yoshiura K, Kanai Y, Ochiai A, Shimoyama Y, Sugimura T, Hirohashi S. Silencing of the E-cadherin invasion-suppressor gene by CpG methylation in human carcinomas. Proc Natl Acad Sci USA. 1995;92:7416-7419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 469] [Cited by in RCA: 469] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 22. | Carvalho B, Pinto M, Cirnes L, Oliveira C, Machado JC, Suriano G, Hamelin R, Carneiro F, Seruca R. Concurrent hypermethylation of gene promoters is associated with a MSI-H phenotype and diploidy in gastric carcinomas. Eur J Cancer. 2003;39:1222-1227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |