Published online Jul 14, 2006. doi: 10.3748/wjg.v12.i26.4175
Revised: October 15, 2005
Accepted: October 26, 2005
Published online: July 14, 2006
AIM: Pancreatic pseudocysts (PPC) as a complication of pancreatitis are approached only in the case of abdominal pain, infection, bleeding, and compression onto the gastrointestinal tract or biliary tree.
METHODS: From 02/01/2002 to 05/31/2004, all consecutive patients with symptomatic PPC who underwent an interventional endoscopic approach were evaluated in this pilot case-series study: Group (Gr.) I-Primary percutaneous (external), ultrasound-guided drainage. Gr. II-Primary EUS-guided cystogastrostomy. Gr. III-EUS-guided cystogastrostomy including intracystic necrosectomy.
RESULTS: (=“follow up”: n = 27): Gr. I (n = 9; 33.3%): No complaints (n = 3); change of an external into an internal drainage (n = 4); complications: (a) bleeding (n = 1) followed by 3 d at ICU, discharge after 40 d; (b) septic shock (n = 1) followed by ICU and several laparotomies for programmed lavage and necrosectomy, death after 74 d. Gr. II (n = 13; 48.1%): No complaints (n = 11); external drainage (n = 2); complications/problems out of the 13 cases: 2nd separate pseudocyst (n = 1) with external drainage (since no communication with primary internal drainage); infection of the residual cyst (n = 1) + following external drainage; spontaneous PPC perforation (n = 1) + following closure of the opening of the cystogastrostomy using clips and subsequently ICU for 2 d. Gr. III (n = 5; 18.5%): No complaints in all patients, in average two endoscopic procedures required (range, 2-6).
CONCLUSION: Interventional endoscopic management of pancreatic pseudocysts is a reasonable alternative treatment option with low invasiveness compared to surgery and an acceptable outcome with regard to the complication rate (11.1%) and mortality (3.7%), as shown by these initial study results.
- Citation: Will U, Wegener C, Graf KI, Wanzar I, Manger T, Meyer F. Differential treatment and early outcome in the interventional endoscopic management of pancreatic pseudocysts in 27 patients. World J Gastroenterol 2006; 12(26): 4175-4178
- URL: https://www.wjgnet.com/1007-9327/full/v12/i26/4175.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i26.4175
Pancreatic pseudocysts (PPC) are complications of acute and chronic pancreatitis[1-4], pancreatic trauma, and pancreatic duct obstruction[4]. For differential diagnosis, PPC needs to be distinguished from cystic tumor lesions of the pancreas. An examination with transabdominal ultrasound and endoscopic ultrasonography (EUS) is considered an initial step of the diagnostic and therapeutic management. PPC are to be treated in the case of subsequent infection, bleeding, and compression onto the gastrointestinal tract or the biliary tree[5]. Abdominal pain is the predominating clinical sign. Fever and septic symptoms are hints for infected PPC. The aim of the study was to demonstrate (1) the differential treatment strategies in PPC[6], (2) the safety of an interventional endoscopic approach of PPC as a reasonable alternative and less invasive treatment option[7,8], and (3) the outcome and short-term follow-up results including problems and complications[7-9].
From February 01, 2002 to May 31, 2004, all consecutive patients with symptomatic PPC were enrolled in this pilot case-series study. Patients underwent an interventional approach in the case of abdominal pain, fever, increase of C-reactive protein (CrP) above the normal range, and compression onto the gastrointestinal tract or the biliary tree caused by the PPC with disturbance(s) of the gastrointestinal passage. Patients were subdivided into 3 groups as shown in Table 1.
| GROUP | Title | n | Indication | Method |
| I | Primary, percutaneous, ultrasound-guided placement of a drainage | 9 | Septic symptoms & inhomogeneous cystic content with suspected infection of PPC revealed by transabdominal ultrasound & EUS | -Ultrasound-guided puncture of the PPC -Aspiration of 10-20 mL of cystic fluid/content -Laboratory analysis (amylase, lipase, CEA, Ca19-9) -Microbiologic investigation for microbial detection/growth -Placement of a 10-Fr. pigtail drainage (Endoflex, Voerde, Germany) -Rinsing with 20-50 mL NaCl/h (bolus) via the drainage -Administration of antibiotics (ceftriaxone [Rocephin®, Hoffmann-La Roche AG, Grenzach-Wyhlen, Germany] plus metronidazol [Clont®, Bayer Vital GmbH, Leverkusen, Germany]) over 7 d |
| II | Primary, EUS-guided cystogastrostomy | 13 | Echo-free PPC with no inner echos & no evidence of infection | -Puncture of the cyst with 19-G needle (Wilson-Cook [Cook Deutschland GmbH], Mönchengladbach, Germany) -Cytologic investigation of cystic fluid/content -Placement of a 8.5-Fr.-double pigtail catheter Endoflex, Voerde, Germany) -Administration of antibiotics (ceftriaxone [Rocephin®, Hoffmann-La Roche AG, Grenzach-Wyhlen, Germany]) over 3 d |
| III | EUS-guided cystogastrostomy with following necrosectomy | 5 | -Suspected sequester & necrosis within the PPC revealed by ultrasound -Persisting fever & septic symptoms after cystogastrostomy & EUS- guided placement of a drainage | -Introduction of a 0.035-Inch guide wire (MTW Endoskopie, Wesel, Germany) via needle in place -Opening of the PPC with needle knife (Erbe Elektromedizin GmbH, Leipzig, Germany) -Enlargement of the PPC opening with balloon dilatation (Boston Scientific Medizintechnik GmbH, Ratingen, Germany) -Endoscopy of the cystic cavity -Removal of the necroses with loop & Dormia´s basket (MTW Endoskopie, Wesel, Germany, each) (min. 2x) -Microbiologic investigation for microbial detection/growth -Placement of a transgastrocystic 8.5-Fr.-double pigtail drainage (Endoflex, Voerde, Germany) -Removal of the pigtail catheter after 3-6 mo -Administration of antibiotics (ceftriaxone plus metronidazol) over 7 d |
A consent form was obtained from each patient enrolled in the study.
Overall, 27 consecutive patients with symptomatic PPC were enrolled in the study over the 28-mo study period. The following details comprise the outcome, subsequent therapeutic steps, and the follow-up data of the various treatment groups.
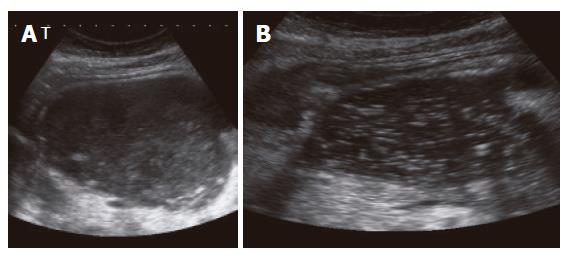
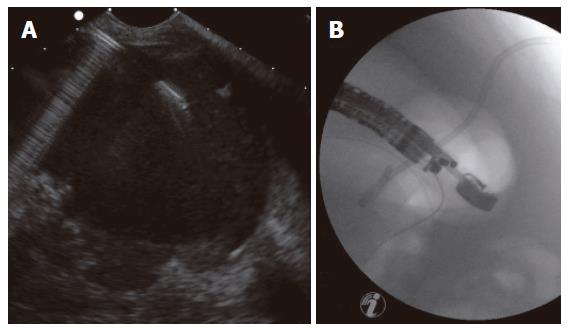
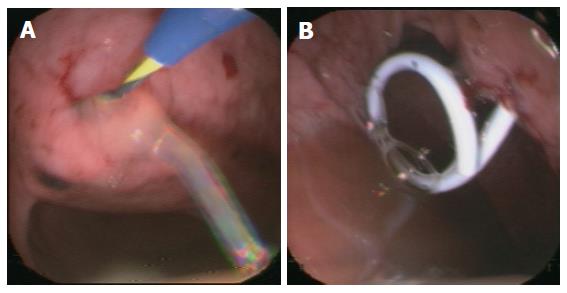
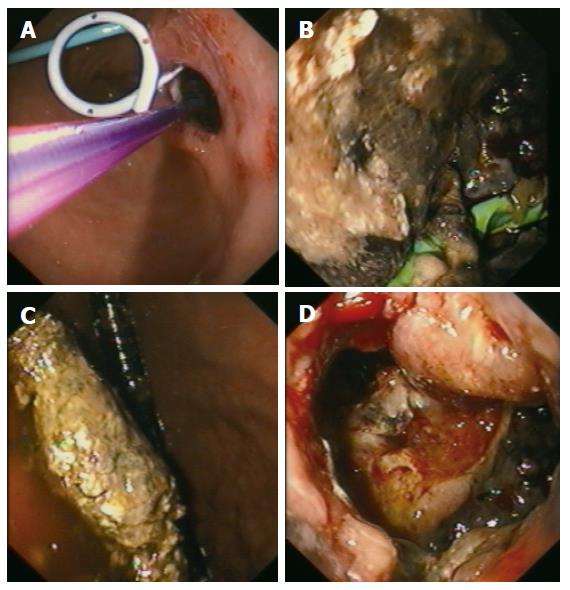
Group I (primary percutaneous, ultrasound-guided placement of a drainage): Nine patients of 27 (33.3%) underwent the approach of an external drainage of the PPC (Figure 1). In three patients, no further complaints were observed with no additional therapy. An internal drainage was placed in four patients (Figures 2 and 3). After the septic symptoms disappeared, the external drainage was replaced by an internal drainage (Figure 4). In the complication profile, there was one case of post-interventional bleeding with a subsequent 3-d stay at the ICU and discharge after a total of 40 d. In one case, septic shock occurred. The patient died after several open laparotomies for programmed lavages and necrectomy after a 74-d care at the ICU.
Group II (primary EUS-guided cystogastrostomy): By the mean of this approach, 13 of 27 patients (48.1%) were treated, aiming for the placement of an internal drainage for the PPC. In the vast majority of patients (n = 11), no further complaints or signs were reported, and no intervention-related symptoms were observed. Therefore, no further therapy was required. In two patients, an additional external drainage was placed: In one of the two patients, additional PPC were found, which, though communicating with other PPC, were not drained by the internally (transgastrocystically) placed drain reaching the originally detected PPC. In the second patient, a subsequent infection of the residual PPC was diagnosed following the initially successful internal drainage. This required a temporary external drainage. Out of 13 patients, there was only one serious complication. During the endoscopic intervention, a spontaneous perforation of the PPC was observed. Therefore, the cystogastrostomy was closed endoscopically by the mean of clips, which resulted in no further complaints, signs and symptoms after a 2-d stay and monitoring at the ICU. Hereafter, the patient´s discharge became possible.
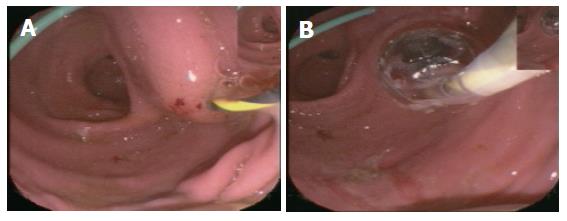
Group III (EUS-guided cystogastrostomy including necrosectomy): This approach was used successfully in 5 of 27 patients (18.5 %) (Figure 5). The postinterventional course of all patients was uneventful and they did not show any problems or complications. On average, two endoscopic procedures (range, 2-6) were required to achieve the intended aim.
All together, there were three serious complications out of 27 subjects who underwent an interventional endoscopic approach of their PPC resulting in a complication rate of 11.1%. However, one patient died post-interventionally (mortality, 3.7%) because of septic complications with the underlying necrotizing pancreatitis.
In this report, treatment results for a novel interventional endoscopic approach for PPC are described. According to the aim of the study, this approach is a reasonable initial option in the therapeutic spectrum and it can be considered an establishing, less invasive, feasible, safe, and effective treatment alternative in experienced hands compared with open surgery, showing acceptable outcome and follow-up results with regard to complication rate and mortality[1-4,9-12]. The great advantage of this endoscopic approach is that it avoids the more severe trauma and invasiveness of a surgical intervention (no general anesthesia, no laparotomy, no risk for wound infection, less alteration of pulmonary and cardiac function, benefit for high-risk patients with a number of accompanying diseases or significant problems in their medical history). This endoscopic approach keeps the surgical intervention option still available in the case of unsuccessful outcome and follow-up. The endoscopic approach may provide comparable complication and mortality rates as shown for open cystogastrostomy or cystojejunostomy in a reasonably selected group of patients. However, the endoscopic approach has not changed but emphasizes the indications and principles[6] for the interventional treatment of PPC as follows: (1) Only symptomatic PPC are to be treated[6,12], (2) Basically, decision-making favors a forced interventional endoscopic approach together with an abdominal surgeon for surgical back-up[6]. (3) EUS is the main tool for diagnostic and treatment of PPC[3,4,6,10,13,14].
In particular, the novel aspect of the suggested approach is the less invasive character of this type of endoscopic intervention. The endoscopic approach includes differential treatment options (primary percutaneous, ultrasound-guided placement of a drainage EUS-guided cystogastrostomy with or without subsequent necrosectomy) and a comparable outcome to open surgery as indicated by periinterventional morbidity and mortality.
The main complications and problems of the endoscopic procedure are subsequent infection of the cyst[6-9], bleeding[3,6-10], pancreatitis[7,8], perforation[9], colonic fistula[15], failure of the drainage[2], conversion[1], PPC recurrence[2,9,10,12], and even death. In our study, we observed only one bleeding, one perforation and one case of septic shock but none of the other possible complications. We showed a complication rate of 11.1% (n = 3), which is relatively low compared to De Palma
et al[7,8] who reported a complication rate of 24.5%. In our study, there was one death due to necrotizing pancreatitis possibly indicating an unsuitable case for the endoscopic procedure.
According to this study, we suggest the following algorithm (a modification of Vosoghi[4] and Baillie[6]): (1) Initially, a possible malignant tumor growth of the cystic lesion needs to be excluded by means of EUS[6], laboratory analysis of cystic contents and cytologic investigation. In the case of an echogenic, thickened cystic wall and detectable Doppler signals within the cystic septum[6], a malignant cystic tumor lesion can be suspected. The suspected diagnosis can be supported by cytologic investigation of cystic content detecting tumor cells. In addition, levels of tumor markers such as CEA and Ca19-9 can be analyzed in the cystic fluid. The sensitivity and specificity of Ca19-9 to predict malignant tumor growth in cystic lesions of the pancreas is approximately 80% (cut off point, 50 000 IU/mL). In the case of a malignant cystic tumor lesion, a surgical approach is obligatory. If there are no hints of malignancy, (2) ERCP follows to clarify whether the PPC communicates with the pancreatic duct[6]. If yes, (3a) a transpapillary drainage needs to be implanted[4,6-10]. Otherwise, (3b) a transgastrocystic, EUS-guided drainage is placed[4,6-10]. If there are hints of an infected PPC or an abscess (echo-rich cystic content, necroses, sequester) revealed by transabdominal ultrasound, EUS, or clinical exam, primarily, (3c) an external (percutaneous) drainage of the PPC is to be favored[2,4]. After placement of a drainage, rinsing the cyst over several days and administration of antibiotics can be initiated. Depending on the patient´s clinical course (persisting PPC of the same size and configuration, persisting septic signs and symptoms, no falling inflammatory laboratory parameters) it has to be decided whether additionally, (3d) a necrosectomy[6] or even (3e) surgical intervention is required[4].
An interesting additional approach to consider is a laparoscopic endogastric pseudocyst gastrostomy, which combines less invasiveness, in particular, in large retrogastric PPC with the potential option of facilitated debridement of necrotic pancreas[1,2].
In conclusion, interventional endoscopic management of pancreatic pseudocysts is a reasonable alternative treatment option considering: (1) surgical principles[6] and (2) lower invasiveness.
Compared with surgical intervention there is a similar or even lower complication rate and mortality as shown by these study results. Subsequent follow-up investigations of the patients treated in this report, favoring again EUS, and studies with a higher value of evidence and larger case numbers are necessary to investigate treatment outcome and possible long-term consequences more appropriately[2,11].
S- Editor Wang J L- Editor Lutze M E- Editor Bi L
| 1. | Ammori BJ, Bhattacharya D, Senapati PS. Laparoscopic endogastric pseudocyst gastrostomy: a report of three cases. Surg Laparosc Endosc Percutan Tech. 2002;12:437-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Bhattacharya D, Ammori BJ. Minimally invasive approaches to the management of pancreatic pseudocysts: review of the literature. Surg Laparosc Endosc Percutan Tech. 2003;13:141-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 75] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 3. | Giovannini M, Binmoeller K, Seifert H. Endoscopic ultrasound-guided cystogastrostomy. Endoscopy. 2003;35:239-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 48] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Vosoghi M, Sial S, Garrett B, Feng J, Lee T, Stabile BE, Eysselein VE. EUS-guided pancreatic pseudocyst drainage: review and experience at Harbor-UCLA Medical Center. MedGenMed. 2002;4:2. [PubMed] |
| 5. | Baillie J. Pancreatic pseudocysts (Part I). Gastrointest Endosc. 2004;59:873-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 73] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 6. | Baillie J. Pancreatic pseudocysts (Part II). Gastrointest Endosc. 2004;60:105-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 39] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | De Palma GD, Galloro G, Puzziello A, Masone S, Diamantis G, Persico G. [Personal experience with the endoscopic treatment of pancreatic pseudocysts. Long-term results and analysis of prognostic factors]. Minerva Chir. 2001;56:475-481. [PubMed] |
| 8. | De Palma GD, Galloro G, Puzziello A, Masone S, Persico G. Endoscopic drainage of pancreatic pseudocysts: a long-term follow-up study of 49 patients. Hepatogastroenterology. 2002;49:1113-1115. [PubMed] |
| 9. | Sharma SS, Bhargawa N, Govil A. Endoscopic management of pancreatic pseudocyst: a long-term follow-up. Endoscopy. 2002;34:203-207. [PubMed] |
| 10. | Dohmoto M, Akiyama K, Lioka Y. Endoscopic and endosonographic management of pancreatic pseudocyst: a long-term follow-up. Rev Gastroenterol Peru. 2003;23:269-275. [PubMed] |
| 11. | Seifert H, Wehrmann T, Schmitt T, Zeuzem S, Caspary WF. Retroperitoneal endoscopic debridement for infected peripancreatic necrosis. Lancet. 2000;356:653-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 209] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 12. | Yang CC, Shin JS, Liu YT, Yueh SK, Chou DA. Management of pancreatic pseudocysts by endoscopic cystogastrostomy. J Formos Med Assoc. 1999;98:283-286. [PubMed] |
| 13. | Norton ID, Clain JE, Wiersema MJ, DiMagno EP, Petersen BT, Gostout CJ. Utility of endoscopic ultrasonography in endoscopic drainage of pancreatic pseudocysts in selected patients. Mayo Clin Proc. 2001;76:794-798. [PubMed] |
| 14. | Seifert H, Faust D, Schmitt T, Dietrich C, Caspary W, Wehrmann T. Transmural drainage of cystic peripancreatic lesions with a new large-channel echo endoscope. Endoscopy. 2001;33:1022-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 52] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Howell DA, Dy RM, Gerstein WH, Hanson BL, Biber BP. Infected pancreatic pseudocysts with colonic fistula formation successfully managed by endoscopic drainage alone: report of two cases. Am J Gastroenterol. 2000;95:1821-1823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |