Published online Jul 7, 2006. doi: 10.3748/wjg.v12.i25.4014
Revised: January 7, 2006
Accepted: January 14, 2006
Published online: July 7, 2006
AIM: To study the condition and potentiality of human umbilical cord blood stem cells (HUCBSC) to differentiate into hepatocytes in vivo or in vitro.
METHODS: In a cell culture study of human umbilical cord blood stem cell (HUCBSC) differentiation, human umbilical cord blood mononuclear cells (HUCBMNC) were separated by density gradient centrifugation. Fibroblast growth factor (FGF) and hepatocyte growth factor (HGF) and the supernatant of fetal liver were added in the inducing groups. Only FGF was added in the control group. The expansion and differentiation of HUCBMNC in each group were observed. Human alpha fetoprotein (AFP) and albumin (ALB) were detected by immunohistochemistry. In the animal experiments, the survival SD rats with acute hepatic injury after carbon tetrachloride (CCL4) injection 48 h were randomly divided into three groups. The rats in group A were treated with human umbilical cord blood serum. The rats in group B were treated with HUCBMNC transplantation. The rats in group C were treated with HUCBMNC transplantation followed by intraperitoneal cyclophosphamide for 7 d. The rats were killed at different time points after the treatment and the liver tissue was histopathologically studied and human AFP and ALB detected by immunohistochemistry. The human X inactive-specific transcript gene fragment in the liver tissue was amplified by PCR to find human DNA.
RESULTS: The results of cell culture showed that adherent cells were stained negative for AFP or ALB in control group. However, the adherent cells in the inducing groups stained positive for AFP or ALB. The result of animal experiment showed that no human AFP or ALB positive cells present in the liver tissue of group A (control group). However, many human AFP or ALB positive cells were scattered around sinus hepaticus and the central veins of hepatic lobules and in the portal area in group B and group C after one month. The fragment of human X chromagene could be detected in the liver tissue of groups B and C, but not in group A.
CONCLUSION: Under certain conditions HUCBSC can differentiate into liver cells in vivo and in vitro.
-
Citation: Tang XP, Zhang M, Yang X, Chen LM, Zeng Y. Differentiation of human umbilical cord blood stem cells into hepatocytes
in vivo andin vitro . World J Gastroenterol 2006; 12(25): 4014-4019 - URL: https://www.wjgnet.com/1007-9327/full/v12/i25/4014.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i25.4014
Umbilical cord blood (UCB) remains in the placenta and umbilical cord after birth. The placenta and umbilical cord blood is usually discarded after delivery as a medical waste. The presence of hematopoietic stem cells (HSC) in UCB was first demonstrated in 1974. There is evidence that UCB is a rich source of HSC. However, it was not until 1989 that experimental and clinical studies were published, indicating that UCB can be used in clinical settings. Since then, UCB has been used as an alternate source of HSC for transplantation. Some data suggest that UCB is a better source of HSC than bone marrow (BM)[1,2]. It has been found that multipotent adult progenitor cells can differentiate into hepatocyte-like cells in vitro[3]. Neurons, astrocytes and oligodendrocytes can be propagated in vitro from UCB cells[4,5], indicating that stem cells can differentiate into hepatocytes[6,7]. These experiments studied the conditions and potentiality of human umbilical cord blood cells (HUCBSC) to differentiate into hepatocyte in vivo or in vitro.
Fetal bovine serum (Hyclone, USA), Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, USA), β-fibroblast growth factor (FGF) and hepatocyte growth factor (HGF) (Preprotech, USA), immunohistochemistry kit (PV900, Beijing), alpha fetoprotein (AFP) and mouse anti human monoclonal antibody (Maixin, Foochow), albumin and (ALB) rabbit anti human polyclonal antibody (DAKO), protease K , NaI, glass powder, Taq DNA polymerase, 10 × buffer, random primer(2.5 mmol/L, dNTP)were provided by Promega Co.
Immediately after birth of the baby, the umbilical cord was cut off and the baby was separated from the placenta and mother. A sterile needle was inserted into the umbilical vein and UCB was drawn into a sterile blood collection bag containing ACD-B or heparin anticoagulant. Once the collection was completed, the specimen was packaged and sent to blood bank for processing and storage at low temperatures. The maternal blood sample was also collected for infectious disease analysis.
Human umbilical cord blood mononuclear cells (HUCBMNC) were separated from UCB by density gradient centrifugation. When cell motility rate was more than 95%, the cell concentration was adjusted to 5 × 105-6/mL.
FGF 2.5 ng/mL and HGF 20 ng/mL and supernatant of fetal liver (5%, V/V) were added into the HUCBMNC culture flasks in the inducing groups. Only FGF was added into the HUCBMNC culture flasks in the control group. The expansion and differentiation of HUCBMNC in each group were observed. Human AFP and ALB were detected by immunohistochemistry.
Adult SD rats, weighing 180 ± 20 g were provided by the Animal Laboratory of the Second Xiangya Hospital. Carbon tetrachloride (CCL4, 0.7 mL/100 g, 10%) was intraperitoneally injected into SD rats to establish an acute hepatic injury model. Surviving rats 48 h after CCL4 injection were randomly divided into three groups. Rats in group A were treated with 1 mL human umbilical cord blood serum. Rats in group B were treated with 1 mL HUCBMNC. Rats in group C were treated with 1 mL HUCBMNC followed by intraperitoneal cyclophosphamide 2 mg/100 g per d for 7 d. The general state of the rats in each group was observed.
The rats were killed at different time points after treatment and the liver tissue was histopathologically studied and detected for human AFP and ALB by immunohisto-chemistry.
The primers of PCR were designed according to human X inactive specific transcript (XIST) gene (upstream primer P1: 5’- TTACTG GCTGTATTGCCTTGC- 3’;downstream primer P2: 5'- ATTATCTCCA CCGCTTCACT -3'). DNA was extracted from the rat liver tissue imbedded in paraffin and amplified by PCR. Gel electrophoresis was performed to find whether human DNA was present in the rat liver tissue.
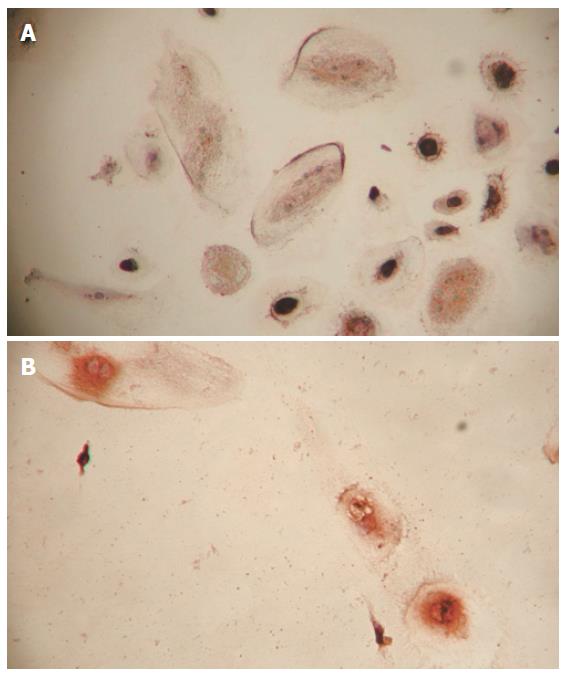
The result showed that most adherent cells had fusiform shape. No polygon shape cells were found in the control group. However, round shape, spherical and polygon shape adherent cells as well as fusiform cells were found in the inducing groups.
The adherent cells in the control group were stained negative for AFP or ALB by immunohistochemistry, thus these adherent cells were most probably fibroblasts. The adherent cells in the inducing groups were stained positive for AFP or ALB by immunohistochemistry (Figures 1A and 1B) and therefore they might be hepatocytes.
Heparin or natrium citricum was used as a decoagulant when UCB was collected. The expansion and differentiation of HUCBMNC anti-coagulated with heparin were better than those of HUCBMNC anti-coagulated with natrium citricum.
Two different inducing factors, HGF and fetal liver supernatant were used to induce differentiation of HUCBSC into hepatocytes. The inducing effect of each was compared. The result showed that the inducing effect of HGF was better than that of fetal liver supematant.
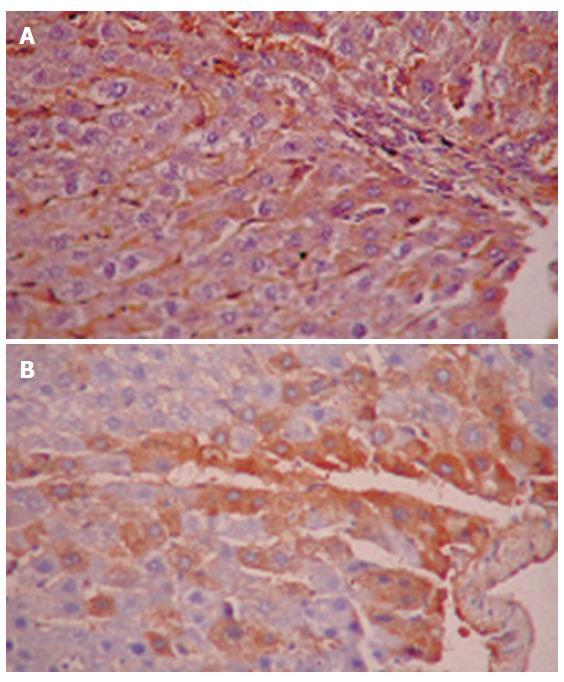
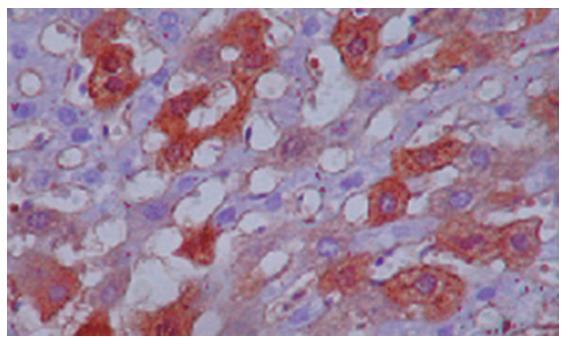
No human AFP or ALB positive cells were found in rat liver tissue of group A (control group) by immunohistochemistry. However, many human AFP or ALB positive cells were scattered around sinus hepaticus and central veins of hepatic lobules and portal area in groups B and C after one month (Figures 2A and 2B). Moreover, a few human ALB positively conjugated nuclear cells were found in rat liver tissue of group C (Figure 3).
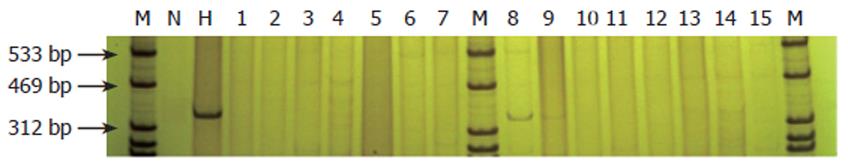
XIST fragment of human X chromagene could be detected in rat liver tissue of groups B and C by DNA extraction and PCR amplification, but no human X chromagene fragment was found in group A (Figure 4).
Stem cells present in adult BM and UCB are undifferentiated long-lived cells that have the ability to proliferate extensively and maintain the ability to differentiate into multiple cell types including bone cells, cartilage cells, fat cells, tendon cells, muscle cells, marrow stromal cells, astrocytes, etc. Potential liver-cell progenitors have been identified from BM, peripheral blood, UCB, fetal liver, adult liver and embryonic stem cells. Differences and similarities are found among cells isolated from rodents and humans[8].
Stem cells are present in UCB and possess several typical traits[9]. Clonal culture of fluorescence-activated cell sorter CD34+ UCB and BM cells revealed a higher incidence of colony-forming cells with greater proliferation capacity in UCB than BM CD34+ cells. UCB CD34+ cells also demonstrated a higher secondary plating efficiency than BM cells. Rats transplanted with UCB mononuclear cells showed significantly higher levels of chimerism than those transplanted with BM mononuclear cells.
Recipients of UCB transplants from HLA-identical siblings have a lower incidence of acute and chronic graft-versus-host disease than recipients of BM transplants from HLA-identical siblings[10]. Immature human UCB cells with high proliferative, replating, ex vivo expansion and mouse NOD/SCID engrafting ability can be stored frozen for > 15 years, and efficiently retrieved, and most likely remain effective for clinical transplantation[11]. Intravenous injection of adult BM cells in FAH-/-mice, an animal model of tyrosinemia type I, could rescue the mice and restore their liver biochemical function. Within BM, only rigorously purified HSCs give rise to donor-derived hematopoietic and hepatic regeneration[12].
Adult human liver cells can be derived from stem cells originating in the BM or circulating outside the liver, so that blood-system stem cells could be used clinically to generate hepatocytes for replacing damaged tissue[13]. BM-derived hepatocyte stem cells integrate with hepatic cell plates and differentiate into mature hepatocytes. In a culture system simulating liver regeneration and containing cholestatic serum, these cells differentiate into mature hepatocytes and metabolize ammonia into urea depending on a yet non-defined humoral signal existing in cholestatic serum. Transmission electron microscopy and three-dimensional digital reconstruction have confirmed hepatocyte ultrastructure of cultured BM-derived hepatocyte stem cells[14]. In a primary culture system supplemented with growth/differentiation factors, about 50% of UCB cells in 21-d cultures express ALB, and ALB+ cells co-express hepatocyte lineage markers. ALB-expressing cells are able to proliferate in the culture system. In the cell-transplantation model of liver-injured SCID mice, inoculated UCB cells develop into functional hepatocytes in the liver, which release human ALB into the sera of the recipient mice. Thus, human UCB is a source of transplantable hepatic progenitor cells[15].
It was reported that human UCB cells can migrate into NOD-SCID liver and become mature hepatocytes. No cell fusion can be detected in any of the human cells found in mouse liver[16]. Human albumin expression can be detected only in CCl4-treated mice receiving transplants of human stem cells, and recovery is increased by administration of human HGF 48 h after CCl4-mediated liver injury[17]. As the cells are morphologically transformed into hepatocyte-like cells, UCB-derived human MSC can express Thy-1, c-Kit and Flt-3 on the cell surface, as well as albumin, AFP and cytokeratin-18 and 19 in the interior. About a half of the cells are found to acquire the ability to transport DiI-Ac-LDL[18].
In this study, UCB transplantation demonstrated a good therapeutic effect on severe viral hepatitis without obvious side effects. UCB attenuated the liver lesions and reproduced hepatocytes. The effect of UCB transplantation combined with plasma exchange (PE) is much better than that of PE alone, suggesting that UCB transplantation can enhance the therapeutic effect of plasma exchange on severe viral hepatitis[19]. After 4 wk of induction of HGF and oncostatin M, stem cells isolated from human BM and UCB showed cuboidal morphology of hepatocytes and cells expressed marker genes specific for liver cells in a time-dependent manner. Differentiated cells have in vitro functions of liver cells, including albumin production, glycogen storage, urea secretion, uptake of low-density lipoprotein, and phenobarbital-inducible cytochrome P450 activity[20]. Human UCB stem cells are able to transdifferentiate into hepatocytes, to improve liver regeneration after damage and mortality rate. Intraperitoneal administration of UCB stem cells contributes to a rapid liver engraftment[21].
CD34+ UCB cells are rich fractions in hepatic progenitor cells, and trans-differentiation from UCB cells into hepatocytes and cell fusion simultaneously occur[22]. UCB stem cell transplantation displays good therapeutic effects on severe viral hepatitis and improves heart injury of the patients. The rat liver immunohistochemistry in this study indicated that UCB stem cells could decrease liver damage and increase hepatocellular regeneration. Human UCB stem cells can differentiate into liver cells in acutely damaged SD rat liver[23]. Xeno-transplantation of human UCB CD34+ (hCBCD34+) cells during pre-immune stages of development in immunocompetent mice might also lead to human-mouse liver chimerism. Freshly isolated hCBCD34 (+) cells were xeno-transplanted into non-immunosuppressed mice by both intra-blastocyst and intra-fetal injections in our study. One and four weeks after birth, immunostaining was carried out for different human-specific hepatocyte markers, such as human hepatocyte-specific antigen, human serum albumin, and human alpha-1-antitrypsin indicated the presence of human hepatocyte-like cells in the livers of transplanted animals. The results indicate that detection of human albumin mRNA further corroborates the development of pre-immune human-mouse chimeras[24].
A new intrinsically pluripotent CD45- population called unrestricted somatic stem cells (USSC) has been isolated from human UCB. These cells grow adherently and can be expanded to at least 1015 cells while maintaining a normal karyotype and a pluripotency including hematopoietic, neural, and hepatic cell differentiation in the noninjured fetal sheep model. More than 20% albumin-producing human parenchymal hepatic cells have been obtained in the absence of cell fusion in this model. One major biological difference between USSCs and human MSC is the ease of generation of USSCs in cytokine-free cultures and the potential to generate hematopoietic cells in vitro. Besides their differentiation potential, USSCs can also be distinguished from MSC by their phenotype. But similar to adult and fetal MSCs, they are also nonimmunogeneic and even immunosuppressive[25].
Some studies have shown that UCB may contain some hepatic progenitors. MSC has been isolated from UCB harboring a broader potential than expected. Single clonally expanded cells isolated from either UCB or BM, can differentiate not only into osteoblasts, adipocytes, and chondrocyte-like cells (as expected for MSCs), but also into different cell types including functional hepatocytes in vitro. When UCB-derived cells are cultured under hepatogenic conditions all cells acquire a cuboidal morphology as opposed to the fibroblast-like morphology of undifferentiated cells. In this study, low level expression of AFP, an early developmental marker gene of hepatoblasts, was detectable by d 7 and remained detectable up to d 35. Expression of cytokeratin-18 and albumin was detectable at all time points, and the expression of tyrosine aminotransferase, a late marker gene of hepatocytes, was detectable by d 14 and increased with time of differentiation. Furthermore, by d 7, differentiated cells were stained positive for albumin. Undifferentiated cells did not express AFP or tyrosine aminotransferase, but expressed low levels of albumin and cytokeratin-18. However, undifferentiated cells were negative for albumin. After 6 wk of differentiation the hepatocyte-like cells demonstrated the ability to take up low-density lipoprotein, a function characteristic of hepatocytes, whereas undifferentiated cells failed to take up low-density lipoprotein[26].
By seeding UCB 2 m-c-Met+ cells (UCBCCs) on the cirrhotic fat-storing cells (CFSC)/HGF feeder layers without any cytokine added, distinct morphological changes in some UCBCCs emerged after co-cultured for 4 d, and the changes increased even more as time went on. Morphological characteristics of hepatocytes occurred after 7 d of coculture in about 38%-46% of UCBCCs, which were identified to possess hepatocyte-like functions. UCBCCs could also differentiate into hepatocyte-like cells by coculturing with CFSC/HGFs separately using trans-wells, although the differentiation efficiency was lower than the former. Moreover, the tests showed limited contribution of control CFSC/neo to the induction, which proved the important sustainment of nonparenchymal cells and extracellular matrices on the stem cell differentiation and prompted us to consider the insufficient factor secretion in nontransfected hepatic stellate cells only[27].
Transplantation of human HSC in sheep has led to the establishment of human hematopoiesis and formation of a significant number of long-lasting, functional human liver cells, with some animals exhibiting levels as high as 20% of donor (human) hepatocytes 11 mo after transplantation. Human hepatocytes generated in sheep retain functional properties of normal hepatocytes, constitute hepatic functional units with the presence of human endothelial and biliary duct cells, and secrete human albumin that is detectable in circulation. Transplanting populations of HSC can efficiently generate a significant number of functional hepatic cells in sheep[28].
Characterization of UCB-derived USSCs with the capacity to differentiate into hematopoietic and nonhematopoietic cells in the absence of cell fusion has highlighted the great potential of stem cell plasticity. A great variety of stem cell types have been defined and even the most pure marrow stem cells are highly heterogeneous. Data suggest that stem cells may exist in a continuum with continually and reversibly changing phenotype[29].
Our study showed that with the induction of HGF or fetal liver supernatant, HUCBSC could expand in vitro and differentiate into polygon cells expressing AFP and ALB. These cells are most likely hepatocytes. Moreover, the expansion and differentiation of HUCBSC anti-coagulated with heparin were better than those of HUCBSC anti-coagulated with natrium citricum, suggesting that natrium citricum may be toxic for HUCBSC or heparin can promote the expansion of HUCBSC in vitro. Therefore, heparin is better than natrium citricum as a UCB collection decoagulant. We also showed that the role of HGF in inducing HUCBSC differentiation into hepatocytes was better than that of fetal liver supernatant, indicating that HGF can promote the expansion of HUCBSC and induce the differentiation of HUCBSC into liver cells. Thus, it is better to use HGF as an inducing factor for the differentiation of HUCBSC.
Our animal experiment showed that after one month treatment with HUCBSC,the rats with acute liver failure were positive for human AFP and ALB in liver tissue. The DNA fragment of human X chromagene could be found in rat liver tissue of HUCBSC-treated group. However, human DNA fragment could not be detected in the control group, indicating that the transfused human ALB and AFP were destroyed in the rats. Therefore, human AFP and ALB detected in rat liver tissue of HUCBSC treated-group were products of liver cells differentiated from HUCBSC. The number of positive human AFP and ALB cells found in HUCBSC-treated groups with or without cyclophosphamide was not obviously different, indicating that immunosuppression has mild or no effect on HUCBSC differentiation in rats. Moreover, a few AFP or human ALB positively conjugated nuclear cells were found in rat liver tissue of HUCBSC-treated group, suggesting that cell fusion and cell division may occur in conjugated nuclear cells.
In conclusion, HUCBSC can differentiate into liver cells in vitro and in vivo under hepatogenic conditions. Stem cells from UCB are able to differentiate into functional hepatocyte-like cells and may serve as a cell source of cell therapy and transplantation for intractable liver diseases.
S- Editor Wang J L- Editor Wang XL E- Editor Ma WH
| 1. | Rocha V, Cornish J, Sievers EL, Filipovich A, Locatelli F, Peters C, Remberger M, Michel G, Arcese W, Dallorso S. Comparison of outcomes of unrelated bone marrow and umbilical cord blood transplants in children with acute leukemia. Blood. 2001;97:2962-2971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 602] [Cited by in RCA: 541] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 2. | Kim DK, Fujiki Y, Fukushima T, Ema H, Shibuya A, Nakauchi H. Comparison of hematopoietic activities of human bone marrow and umbilical cord blood CD34 positive and negative cells. Stem Cells. 1999;17:286-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 61] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Schwartz RE, Reyes M, Koodie L, Jiang Y, Blackstad M, Lund T, Lenvik T, Johnson S, Hu WS, Verfaillie CM. Multipotent adult progenitor cells from bone marrow differentiate into functional hepatocyte-like cells. J Clin Invest. 2002;109:1291-1302. [PubMed] |
| 4. | Buzańska L, Machaj EK, Zabłocka B, Pojda Z, Domańska-Janik K. Human cord blood-derived cells attain neuronal and glial features in vitro. J Cell Sci. 2002;115:2131-2138. [PubMed] |
| 5. | Brazelton TR, Rossi FM, Keshet GI, Blau HM. From marrow to brain: expression of neuronal phenotypes in adult mice. Science. 2000;290:1775-1779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1260] [Cited by in RCA: 1177] [Article Influence: 47.1] [Reference Citation Analysis (0)] |
| 6. | Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, Boggs SS, Greenberger JS, Goff JP. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284:1168-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1795] [Cited by in RCA: 1669] [Article Influence: 64.2] [Reference Citation Analysis (0)] |
| 7. | Fiegel HC, Lioznov MV, Cortes-Dericks L, Lange C, Kluth D, Fehse B, Zander AR. Liver-specific gene expression in cultured human hematopoietic stem cells. Stem Cells. 2003;21:98-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 97] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 8. | Laurson J, Selden C, Hodgson HJ. Hepatocyte progenitors in man and in rodents--multiple pathways, multiple candidates. Int J Exp Pathol. 2005;86:1-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Kim JW, Kim SY, Park SY, Kim YM, Kim JM, Lee MH, Ryu HM. Mesenchymal progenitor cells in the human umbilical cord. Ann Hematol. 2004;83:733-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 90] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 10. | Rocha V, Wagner JE Jr, Sobocinski KA, Klein JP, Zhang MJ, Horowitz MM, Gluckman E. Graft-versus-host disease in children who have received a cord-blood or bone marrow transplant from an HLA-identical sibling. Eurocord and International Bone Marrow Transplant Registry Working Committee on Alternative Donor and Stem Cell Sources. N Engl J Med. 2000;342:1846-1854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 664] [Cited by in RCA: 589] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 11. | Broxmeyer HE, Srour EF, Hangoc G, Cooper S, Anderson SA, Bodine DM. High-efficiency recovery of functional hematopoietic progenitor and stem cells from human cord blood cryopreserved for 15 years. Proc Natl Acad Sci U S A. 2003;100:645-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 149] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 12. | Lagasse E, Connors H, Al-Dhalimy M, Reitsma M, Dohse M, Osborne L, Wang X, Finegold M, Weissman IL, Grompe M. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat Med. 2000;6:1229-1234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1764] [Cited by in RCA: 1631] [Article Influence: 65.2] [Reference Citation Analysis (0)] |
| 13. | Alison MR, Poulsom R, Jeffery R, Dhillon AP, Quaglia A, Jacob J, Novelli M, Prentice G, Williamson J, Wright NA. Hepatocytes from non-hepatic adult stem cells. Nature. 2000;406:257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 779] [Cited by in RCA: 749] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 14. | Avital I, Inderbitzin D, Aoki T, Tyan DB, Cohen AH, Ferraresso C, Rozga J, Arnaout WS, Demetriou AA. Isolation, characterization, and transplantation of bone marrow-derived hepatocyte stem cells. Biochem Biophys Res Commun. 2001;288:156-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 175] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 15. | Kakinuma S, Tanaka Y, Chinzei R, Watanabe M, Shimizu-Saito K, Hara Y, Teramoto K, Arii S, Sato C, Takase K. Human umbilical cord blood as a source of transplantable hepatic progenitor cells. Stem Cells. 2003;21:217-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 145] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 16. | Newsome PN, Johannessen I, Boyle S, Dalakas E, McAulay KA, Samuel K, Rae F, Forrester L, Turner ML, Hayes PC. Human cord blood-derived cells can differentiate into hepatocytes in the mouse liver with no evidence of cellular fusion. Gastroenterology. 2003;124:1891-1900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 200] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 17. | Wang X, Ge S, McNamara G, Hao QL, Crooks GM, Nolta JA. Albumin-expressing hepatocyte-like cells develop in the livers of immune-deficient mice that received transplants of highly purified human hematopoietic stem cells. Blood. 2003;101:4201-4208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 179] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 18. | Hong SH, Gang EJ, Jeong JA, Ahn C, Hwang SH, Yang IH, Park HK, Han H, Kim H. In vitro differentiation of human umbilical cord blood-derived mesenchymal stem cells into hepatocyte-like cells. Biochem Biophys Res Commun. 2005;330:1153-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 168] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 19. | Tang XP, Yang X, Tan H, Ding YL, Zhang M, Wang WL. Clinical and experimental study on therapeutic effect of umbilical cord blood transplantation on severe viral hepatitis. World J Gastroenterol. 2003;9:1999-2003. [PubMed] |
| 20. | Lee KD, Kuo TK, Whang-Peng J, Chung YF, Lin CT, Chou SH, Chen JR, Chen YP, Lee OK. In vitro hepatic differentiation of human mesenchymal stem cells. Hepatology. 2004;40:1275-1284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 668] [Cited by in RCA: 659] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 21. | Di Campli C, Piscaglia AC, Pierelli L, Rutella S, Bonanno G, Alison MR, Mariotti A, Vecchio FM, Nestola M, Monego G. A human umbilical cord stem cell rescue therapy in a murine model of toxic liver injury. Dig Liver Dis. 2004;36:603-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 60] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 22. | Tanabe Y, Tajima F, Nakamura Y, Shibasaki E, Wakejima M, Shimomura T, Murai R, Murawaki Y, Hashiguchi K, Kanbe T. Analyses to clarify rich fractions in hepatic progenitor cells from human umbilical cord blood and cell fusion. Biochem Biophys Res Commun. 2004;324:711-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Tang XP, Yang X, Zhang M, Wang WL, Chen LM. [Clinical and experimental study of the therapeutic effect of umbilical cord blood stem cell transplantation on liver failure and heart damage in severe viral hepatitis patients]. Zhonghua Gan Zang Bing Za Zhi. 2005;13:259-263. [PubMed] |
| 24. | Turrini P, Monego G, Gonzalez J, Cicuzza S, Bonanno G, Zelano G, Rosenthal N, Paonessa G, Laufer R, Padron J. Human hepatocytes in mice receiving pre-immune injection with human cord blood cells. Biochem Biophys Res Commun. 2005;326:66-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Gilgenkrantz H. Mesenchymal stem cells: an alternative source of hepatocytes. Hepatology. 2004;40:1256-1259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Lee OK, Kuo TK, Chen WM, Lee KD, Hsieh SL, Chen TH. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood. 2004;103:1669-1675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 999] [Cited by in RCA: 950] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 27. | Wang Y, Nan X, Li Y, Zhang R, Yue W, Yan F, Pei X. Induction of umbilical cord blood-derived beta2m-c-Met+ cells into hepatocyte-like cells by coculture with CFSC/HGF cells. Liver Transpl. 2005;11:635-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Almeida-Porada G, Porada CD, Chamberlain J, Torabi A, Zanjani ED. Formation of human hepatocytes by human hematopoietic stem cells in sheep. Blood. 2004;104:2582-2590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 97] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 29. | Quesenberry PJ, Dooner G, Colvin G, Abedi M. Stem cell biology and the plasticity polemic. Exp Hematol. 2005;33:389-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |